Abstract
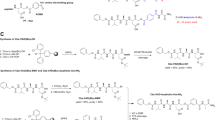
This protocol describes the gram-scale solution-phase synthesis of the colorimetric caspase-3/7 substrate Ac-DEVD-pNA. The caspase enzymes are integral to cellular inflammation and apoptotic cascades, and are commonly studied by cell biologists, medicinal chemists and chemical biologists. In particular, the assessment of caspase enzymatic activity is a standard method to evaluate cell death pathways and new apoptosis-modulating agents. Caspase enzymatic activity can be conveniently monitored with peptidic chromogenic or fluorogenic substrates, with certain peptide sequences imparting selectivity for certain caspases. The synthesis of these peptide substrates is typically carried out by solid-phase synthesis, a method that is not ideal for production of the gram quantities needed for high-throughput screening. Described herein is a facile method for the synthesis of the Ac-DEVD-pNA caspase-3/7 substrate using solution-phase peptide synthesis. This protocol, involving iterative PyBOP-mediated couplings and Fmoc deprotections, is rapid (about 5 d), operationally simple and can be used to generate over 1 g of product at a fraction of the cost of the commercial substrate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 9, 459–470 (2002).
Bose, K., Pop, C., Feeney, B. & Clark, A.C. An uncleavable procaspase-3 mutant has a lower catalytic efficiency but an active site similar to that of mature caspase-3. Biochemistry 42, 12298–12310 (2003).
Hengartner, M.O. The biochemistry of apoptosis. Nature 407, 770–776 (2000).
Acehan, D. et al. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell 9, 423–432 (2002).
Chao, Y. et al. Engineering a dimeric caspase-9: a re-evaluation of the induced proximity model for caspase activation. PLoS Biol. 3, e183 (2005).
Yin, Q. et al. Caspase-9 holoenzyme is a specific and optimal procaspase-3 processing machine. Mol. Cell 22, 259–268 (2006).
Walsh, J.G. et al. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. USA 105, 12815–12819 (2008).
Riedl, S.J. et al. Structural basis for the activation of human procaspase-7. Proc. Natl. Acad. Sci. USA 98, 14790–14795 (2001).
Luthi, A.U. & Martin, S.J. The CASBAH: a searchable database of caspase substrates. Cell Death Differ. 14, 641–650 (2007).
Timmer, J.C. & Salvesen, G.S. Caspase substrates. Cell Death Differ. 14, 66–72 (2007).
Engidawork, E., Gulesserian, T., Yoo, B.C., Cairns, N. & Lubec, G. Alteration of caspases and apoptosis-related proteins in brains of patients with Alzheimer's disease. Biochem. Biophys. Res. Commun. 281, 84–93 (2001).
Blandini, F. et al. Peripheral proteasome and caspase activity in Parkinson disease and Alzheimer disease. Neurology 66, 529–534 (2006).
Roth, K.A. Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J. Neuropathol. Exp. Neurol. 60, 829–838 (2001).
Nagatsu, T. & Sawada, M. Biochemistry of postmortem brains in Parkinson's disease: historical overview and future prospects. J. Neural. Transm. Suppl. 113–120 (2007).
Rubinsztein, D.C. & Carmichael, J. Huntington's disease: molecular basis of neurodegeneration. Expert Rev. Mol. Med. 5, 1–21 (2003).
Martin, L.J. Neuronal death in amyotrophic lateral sclerosis is apoptosis: possible contribution of a programmed cell death mechanism. J. Neuropathol. Exp. Neurol. 58, 459–471 (1999).
Green, D.R. & Kroemer, G. Pharmacological manipulation of cell death: clinical applications in sight? J. Clin. Invest. 115, 2610–2617 (2005).
Hanahan, D. & Weinberg, R.A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Soung, Y.H. et al. Caspase-8 gene is inactivated by somatic mutations in gastric carcinomas. Cancer Res. 65, 815–821 (2005).
Soung, Y.H. et al. Caspase-8 gene is frequently inactivated by the frameshift somatic mutation 1225_1226delTG in hepatocellular carcinomas. Oncogene 24, 141–147 (2005).
Kim, H.S. et al. Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology 125, 708–715 (2003).
Mandruzzato, S., Brasseur, F., Andry, G., Boon, T. & van der Bruggen, P. A CASP-8 mutation recognized by cytolytic T lymphocytes on a human head and neck carcinoma. J. Exp. Med. 186, 785–793 (1997).
Teitz, T. et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat. Med. 6, 529–535 (2000).
Teitz, T., Lahti, J.M. & Kidd, V.J. Aggressive childhood neuroblastomas do not express caspase-8: an important component of programmed cell death. J. Mol. Med. 79, 428–436 (2001).
Shin, M.S. et al. Inactivating mutations of CASP10 gene in non-Hodgkin lymphomas. Blood 99, 4094–4099 (2002).
Park, W.S. et al. Inactivating mutations of the caspase-10 gene in gastric cancer. Oncogene 21, 2919–2925 (2002).
Kania, J., Konturek, S.J., Marlicz, K., Hahn, E.G. & Konturek, P.C. Expression of survivin and caspase-3 in gastric cancer. Dig. Dis. Sci. 48, 266–271 (2003).
Zou, H. et al. Regulation of the Apaf-1/caspase-9 apoptosome by caspase-3 and XIAP. J. Biol. Chem. 278, 8091–8098 (2003).
Shiozaki, E.N. et al. Mechanism of XIAP-mediated inhibition of caspase-9. Mol. Cell 11, 519–527 (2003).
Roy, S. et al. Maintenance of caspase-3 proenzyme dormancy by an intrinsic 'safety catch' regulatory tripeptide. Proc. Natl. Acad. Sci. USA 98, 6132–6137 (2001).
Grigoriev, M.Y., Pozharissky, K.M., Hanson, K.P., Imyanitov, E.N. & Zhivotovsky, B. Expression of caspase-3 and -7 does not correlate with the extent of apoptosis in primary breast carcinomas. Cell Cycle 1, 337–342 (2002).
Krepela, E., Prochazka, J., Liul, X., Fiala, P. & Kinkor, Z. Increased expression of Apaf-1 and procaspase-3 and the functionality of intrinsic apoptosis apparatus in non-small cell lung carcinoma. Biol. Chem. 385, 153–168 (2004).
Fink, D. et al. Elevated procaspase levels in human melanoma. Melanoma Res. 11, 385–393 (2001).
Estrov, Z. et al. Caspase 2 and caspase 3 protein levels as predictors of survival in acute myelogenous leukemia. Blood 92, 3090–3097 (1998).
Putt, K.S. et al. Small molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nat. Chem. Biol. 2, 543–550 (2006).
Peterson, Q.P. et al. PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition. J. Mol. Biol. 388, 144–158 (2009).
Peterson, Q.P. et al. Procaspase-3 activation as an anti-cancer strategy: structure-activity relationship of PAC-1, and its cellular co-localization with procaspase-3. J. Med. Chem. 52, 5721–5731 (2009).
Thornberry, N. et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Funtional relationships established for key mediators of apoptosis. J. Biol. Chem. 27, 17907–17911 (1997).
Nicholson, D.W. et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376, 37–43 (1995).
Goode, D.R., Sharma, A.K. & Hergenrother, P.J. Using peptidic inhibitors to systematically probe the S1' site of caspase-3 and caspase-7. Org. Lett. 7, 3529–3532 (2005).
Smolewski, P., Grabarek, J., Phelps, D.J. & Darzynkiewicz, Z. Stathmo-apoptosis: arresting apoptosis by fluorochrome-labeled inhibitor of caspases. Int. J. Oncol. 19, 657–663 (2001).
Hardy, J.A. & Wells, J.A. Dissecting an allosteric switch in caspase-7 using chemical and mutational probes. J. Biol. Chem. 284, 26063–26069 (2009).
Chu, W., Rothfuss, J., Chu, Y., Zhou, D. & Mach, R.H. Synthesis and in vitro evaluation of sulfonamide isatin Michael acceptors as small molecule inhibitors of caspase-6. J. Med. Chem. 52, 2188–2191 (2009).
Chu, W. et al. Isatin sulfonamide analogs containing a Michael addition acceptor: a new class of caspase 3/7 inhibitors. J. Med. Chem. 50, 3751–3755 (2007).
Chu, W. et al. N-benzylisatin sulfonamide analogues as potent caspase-3 inhibitors: synthesis, in vitro activity, and molecular modeling studies. J. Med. Chem. 48, 7637–7647 (2005).
Guo, Z., Xian, M., Zhang, W., McGill, A. & Wang, P.G. N-nitrosoanilines: a new class of caspase-3 inhibitors. Bioorg. Med. Chem. 9, 99–106 (2001).
Chen, Y.-H. et al. Design, synthesis and biological evaluation of isoquinoline-1,3,4-trione derivatives as potent caspase-3 inhibitors. J. Med. Chem. 49, 1613–1623 (2006).
Lee, D. et al. Potent and selective non-peptide inhibitors of caspases 3 and 7. J. Med. Chem. 44, 2015–2026 (2001).
Wu, J.C. & Fritz, L.C. Irreversible caspase inhibitors: tools for studying apoptosis. Methods 17, 320–328 (1999).
Chapman, J.G. et al. A novel nonpeptidic caspase-3/7 inhibitor, (S)-(+)-5-[1-(2-methoxymethylpyrrolidinyl)sulfonyl]isatin reduces myocardial ischemic injury. Eur. J. Pharmacol. 456, 59–68 (2002).
Hardy, J.A., Lam, J., Nguyen, J.T., O'Brien, T. & Wells, J.A. Discovery of an allosteric site in the caspases. Proc. Natl. Acad. Sci. USA 101, 12461–12466 (2004).
Hoeg-Jensen, T., Jakobsen, M.H. & Holm, A. A new method for rapid solution synthesis of shorter peptides by use of PyBOP. Tetrahedron Lett. 32, 6387–6390 (1991).
Carpino, L.A. et al. Rapid, continuous solution-phase peptide synthesis: application to peptides of pharmaceutical interest. Org. Process Res. Dev. 7, 28–37 (2002).
Thompson, C.M., Quinn, C.A. & Hergenrother, P.J. Total synthesis and cytoprotective properties of dykellic acid. J. Med. Chem. 52, 117–125 (2009).
Rijkers, D.T.S., Adams, H.P.H.M., Hemker, H.C. & Tesser, G.I. A convenient synthesis of amino acid p-nitroanilides; synthons in the synthesis of protease substrates. Tetrahedron 51, 11235–11250 (1995).
Acknowledgements
We are grateful to the National Institutes of Health (R01-CA120439) for the support of this work. Q.P.P. was partially supported by a Chemistry–Biology Interface Training Grant from the National Institutes of Health (Ruth L. Kirschstein National Research Service Award 1 T32 GM070421 from the National Institute of General Medical Sciences) and by a predoctoral fellowship from the ACS Division of Medicinal Chemistry. D.C.W. was partially supported by Ruth L. Kirschstein National Research Service Award 3F31CA130138-01S1.
Author information
Authors and Affiliations
Contributions
All authors contributed extensively to the work presented in this paper.
Corresponding author
Supplementary information
Supplementary Data
NMR Spectrum for Ac-DEVD-pNA (PDF 123 kb)
Rights and permissions
About this article
Cite this article
Peterson, Q., Goode, D., West, D. et al. Preparation of the caspase-3/7 substrate Ac-DEVD-pNA by solution-phase peptide synthesis. Nat Protoc 5, 294–302 (2010). https://doi.org/10.1038/nprot.2009.223
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.223
This article is cited by
-
Influence of amino acid and N-terminal protection residue structures on peptide p-nitroanilide adsorption on polystyrene-based support
Amino Acids (2023)
-
A synthesized olean-28,13β-lactam targets YTHDF1-GLS1 axis to induce ROS-dependent metabolic crisis and cell death in pancreatic adenocarcinoma
Cancer Cell International (2022)
-
A miRNA-101-3p/Bim axis as a determinant of serum deprivation-induced endothelial cell apoptosis
Cell Death & Disease (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.