Abstract
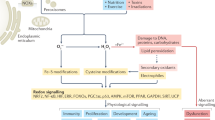
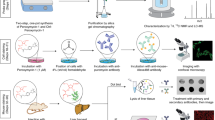
Cells contain a large number of antioxidants to prevent or repair the damage caused by reactive oxygen species, as well as to regulate redox-sensitive signaling pathways. General protocols are described to measure the antioxidant enzyme activity of superoxide dismutase (SOD), catalase and glutathione peroxidase. The SODs convert superoxide radical into hydrogen peroxide and molecular oxygen, whereas the catalase and peroxidases convert hydrogen peroxide into water. In this way, two toxic species, superoxide radical and hydrogen peroxide, are converted to the harmless product water. Western blots, activity gels and activity assays are various methods used to determine protein and activity in both cells and tissue depending on the amount of protein required for each assay. Other techniques including immunohistochemistry and immunogold can further evaluate the levels of the various antioxidant enzymes in tissues and cells. In general, these assays require 24–48 h to complete.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cerutti, P.A. Prooxidant states and cancer. Science 227, 375–381 (1985).
Allen, R.G. & Balon, A.K. Oxidative influence in development and differentiation: an overview of a free radical theory of development. Free Radic. Biol. Med. 6, 631–661 (1989).
Vaquero, E.C., Edderkaoui, M., Pandol, S.J., Gukovsky, I. & Gukovskaya, A.S. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J. Biol. Chem. 27, 34643–34654 (2004).
Shibanuma, M., Kuroki, T. & Nose, K. Induction of DNA replication and expression of proto-oncogenes c-myc and c-fos in quiescent Balb/3T3 cells by xanthine/xanthine oxidase. Oncogene 3, 17–21 (1988).
Lo, Y.Y.C., Wong, J.M.S. & Cruz, T.F. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J. Biol. Chem. 271, 15703–15707 (1996).
McCord, J.M., Keele, B.B. & Fridovich, I. An enzyme based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc. Natl. Acad. Sci. USA 68, 1024–1027 (1971).
Liu, J. et al. Redox regulation of pancreatic cancer cell growth: role of glutathione peroxidase in the suppression of the malignant phenotype. Hum. Gene Ther. 15, 239–250 (2004).
Teoh, M.L.T. et al. Modulation of reactive oxygen species (ROS) in pancreatic cancer: insight into tumor growth suppression by the superoxide dismutases. Clin. Cancer Res. 13, 7441–7450 (2007).
Okado-Matsumoto, A. & Fridovich, I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J. Biol. Chem. 27, 38388–38393 (2001).
Sturtz, L.A., Diekert, K., Jensen, L.T., Lill, R. & Culotta, V.C. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 276, 38084–38089 (2001).
Marklund, S.L., Holme, E. & Hellner, L. Superoxide dismutase in extracellular fluids. Clin. Chim. Acta 126, 41–51 (1982).
Marklund, S. Human copper-containing superoxide dismutase of high molecular weight. Proc. Natl. Acad. Sci. USA 79, 7634–7638 (1982).
Sandstrom, J., Carlsson, L., Marklund, S.L. & Edlund, T. The heparin-binding domain of extracellular superoxide dismutase C and formation of variants with reduced heparin affinity. J. Biol. Chem. 267, 18205–18209 (1992).
Wong, H.W.G., Elwell, J.H., Oberley, L.W. & Goeddel, D.V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell 58, 923–931 (1989).
Cullen, J.J. et al. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer Res. 63, 1297–1303 (2003).
Weydert, C. et al. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol. Cancer Ther. 2, 361–369 (2003).
Ough, M. et al. Inhibition of cell growth by overexpression of manganese superoxide dismutase (MnSOD) in human pancreatic carcinoma. Free Radic. Res. 38, 1223–1233 (2004).
Nishikawa, M., Tamada, A., Kumai, H., Yamashita, F. & Hashida, M. Inhibition of experimental pulmonary metastasis by controlling biodistribution of catalase in mice. Int. J. Cancer 99, 474–479 (2002).
Nelson, K.K. et al. Elevated Sod2 activity augments matrix metalloproteinase expression: evidence for the involvement of endogenous hydrogen peroxide in regulating metastasis. Clin. Cancer Res. 9, 424–432 (2003).
Mills, G.C. Glutathione peroxidase, an erythrocyte enzyme that protects hemoglobin from oxidative damage. J. Biol. Chem. 229, 189–197 (1957).
Hann, J.B. et al. Mice with homozygous null mutation for the most abundant glutathione peroxidase, GPx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J. Biol. Chem. 273, 22528–22536 (1998).
Mirault, M.E., Tremblay, A., Beaudoin, N. & Tremblay, M. Overexpression of seleno-glutathione peroxidase by gene transfer enhances the resistance of T47D human breast cells to clastogenic oxidants. J. Biol. Chem. 266, 20572–20760 (1991).
Kelner, M.J., Bagnell, R.D., Uglik, S.F., Montoya, M.A. & Mullenbach, G.T. Heterologous expression of selenium-dependent glutathione peroxidase afford cellular resistance to paraquat. Arch. Biochem. Biophys. 323, 40–46 (1995).
Hockenbery, D.M., Oltvai, Z.N., Yin, X., Milliman, C.L. & Korsmeyer, S.J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75, 241–251 (1993).
Kayanoki, Y. et al. The protective role of glutathione peroxidase in apoptosis induced by reactive oxygen species. Biochemistry 119, 817–822 (1996).
Lewis, A. et al. Metastatic progression of pancreatic cancer: changes in antioxidant enzymes and cell growth. Clin. Exp. Metastasis 22, 523–532 (2006).
Czaja, M.J. et al. Induction of MnSOD gene expression in a hepatic model of TNF-α toxicity does not result in increased protein. Am. J. Physiol. 266, G737–G744 (1994).
Cullen, J.J., Mitros, F.A. & Oberley, L.W. Expression of antioxidant enzymes in diseases of the human pancreas: another link between chronic pancreatitis and pancreatic cancer. Pancreas 26, 23–27 (2003).
Lewis, A. et al. Targeting NAD(P)H:quinone oxidoreductase (NQO1) in pancreatic cancer. Mol. Carcinog. 43, 215–224 (2005).
Du, J. et al. Mitochondrial production of reactive oxygen species mediate dicumarol-induced cytotoxicity in cancer cells. J. Biol. Chem. 281, 37416–37426 (2006).
Oberley, L.W. & Spitz, D.R. Nitroblue tetrazolium. In Handbook of Methods for Oxygen Radical Research (ed. Greenwald, R.A.) 217–220 (CRC Press, Boca Raton, Florida, 1985).
Spitz, D.R. & Oberley, L.W. An assay for superoxide dismutase in mammalian tissue homogenates. Anal. Biochem. 179, 8–18 (1989).
Lowry, O.H., Rosebrough, N.J. & Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Burton, K. A study of the conditions and mechanisms of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 62, 215–223 (1956).
Crapo, J.D., Oury, T., Rabouille, C., Slot, J.W. & Chang, L.Y. Copper, zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc. Natl. Acad. Sci. USA 89, 10405–10409 (1992).
Spitz, D.R. & Oberley, L.W. Measurement of MnSOD and CuZnSOD activity in mammalian tissue homogenates. Curr. Protoc. Toxicol. 8, 7.5.1–7.5.11 (2001).
Weiss, R.H. et al. Evaluation of activity of putative superoxide dismutase mimics: direct analysis by stopped-flow kinetics. J. Biol. Chem. 268, 23049–23054 (1993).
Ornstein, L. Disc electrophoresis. I. Background and theory. Ann. N.Y. Acad. Sci. 121, 321–349 (1964).
Davis, B.J. Disc electrophoresis-II. Method and application to human serum proteins. Ann. N.Y. Acad. Sci. 121, 404–427 (1964).
Beauchamp, C. & Fridovich, I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287 (1971).
Beers, R.F. Jr. & Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195, 133–140 (1952).
Aebi, H. & Lester, P. Catalase in vitro. In Methods in Enzymology. Vol. 105 (ed. Packer, L.) 121–126 (Academic Press, New York, New York, 1984).
Treadwell, F.P. & Hall, W.T. Analytical chemistry. In Analytical Chemistry. Based on the German text by F.P. Treadwell edn. 9, Vol. 2 (ed. Hall, W.T.) (John Wiley & Sons, New York, New York, 1948).
Paglia, P.E. & Valentine, W.N. Studies on the quantitation and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70, 158–169 (1967).
Liu, J. et al. Suppression of the malignant phenotype in pancreatic cancer by the overexpression of phospholipid hydroperoxide glutathione peroxidase (PhGPx). Hum. Gene Ther. 17, 105–116 (2006).
Günzler, W.A. & Flohé, L. Glutathione peroxidase. In Handbook of Methods for Oxygen Radical Research (ed. Greenwald, R.A.) 285–290 (CRC Press, Boca Raton, Florida, 1985).
Lawrence, R.A. & Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys. Res. Commun. 71, 952–958 (1976).
Lam, E.W.N. et al. Immunolocalization and adenoviral vector-mediated manganese superoxide dismutase gene transfer to experimental oral tumors. J. Dent. Res. 79, 1410–1417 (2000).
Acknowledgements
This work was supported by an NIH Grant CA137230 and a VA Merit Review Grant.
Author information
Authors and Affiliations
Contributions
C.J.W. and J.J.C. discussed and commented on the paper at all stages.
Corresponding author
Supplementary information
Supplementary Method
Method for fixing tissues and cultured cells for immunogold immunohistochemistry. (DOC 41 kb)
Rights and permissions
About this article
Cite this article
Weydert, C., Cullen, J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5, 51–66 (2010). https://doi.org/10.1038/nprot.2009.197
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.197
This article is cited by
-
Functional characterization of a manganese superoxide dismutase from Avicennia marina: insights into its role in salt, hydrogen peroxide, and heavy metal tolerance
Scientific Reports (2024)
-
From microbes to molecules: a review of microbial-driven antioxidant peptide generation
World Journal of Microbiology and Biotechnology (2024)
-
Ameliorative Effects of Zn and Se Supplementation on Heavy Metal Mixture Burden via Increased Renal Metal Excretion and Restoration of Redoxo-Inflammatory Alterations
Biological Trace Element Research (2024)
-
Assessment of Oxidative Liver Injury Caused by Cashew Nut Shell Liquid (CNSL) via Interacting with Bcl2 Gene in Male Wistar Rats and Basic In-silico Approaches
Proceedings of the Zoological Society (2024)
-
Protection of Arsenic Induced Oxidative Stress in Reproductive Organs and Female Infertility by Pectic Polysaccharide of Momordica charantia: An In Vivo Study in dose Dependent Manner
Proceedings of the Zoological Society (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.