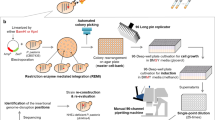
Abstract
Methods development in chromatography is a time-consuming, trial-and-error process that requires laborious experimentation. We describe a high-throughput screening (HTS) protocol for the rapid identification of chromatographic steps for protein purification from cell-free expression broths. Broths containing the protein are loaded on different chromatographic resins aliquotted in membrane-bottomed microtiter plates. Serial step elution of protein from resins results in fraction collection in 96-well plates. Choice of the optimal chromatographic operating conditions is based on protein purity in eluted fractions, determined using SDS-PAGE analysis or similar analytical techniques. The screening procedure is then repeated in order to identify the subsequent chromatographic steps, ultimately leading to high purities of the protein. The protocol takes ∼24 h in order to determine the required sequence of chromatographic steps. The use of a miniaturized screen facilitates screening of a range of media and operating conditions (i.e., pH, salt concentration, and so on.) in parallel and is a novel approach to chromatographic methods development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
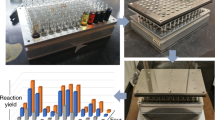
References
Sunasara, K.M., Xia, F., Gronke, R.S. & Cramer, S.M. Application of hydrophobic interaction displacement chromatography for an industrial protein purification. Biotechnol. Bioeng. 82, 330–339 (2003).
Shepard, S.R. et al. Large-scale purification of recombinant human angiostatin. Protein Expr. Purif. 20, 216–227 (2000).
Prazeres, D.M., Schluep, T. & Cooney, C. Preparative purification of supercoiled plasmid DNA using anion-exchange chromatography. J. Chromatogr. A 806, 31–45 (1998).
Haldankar, R., Kopchick, J.J. & Ridgway, D. Stable production of a human growth hormone antagonist from CHO cells adapted to serum-free suspension culture. Biotechnol. Prog. 15, 336–346 (1999).
Shukla, A.A. et al. Preparative purification of a recombinant protein by hydrophobic interaction chromatography: modulation of selectivity by the use of chaotropic additives. Biotechnol. Prog. 18, 556–564 (2002).
Levison, P.R. Large-scale ion-exchange column chromatography of proteins. Comparison of different formats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 790, 17–33 (2003).
Van Hijfte, L., Marciniak, G. & Froloff, N. Combinatorial chemistry, automation and molecular diversity: new trends in the pharmaceutical industry. J. Chromatogr. B Biomed. Sci. Appl. 725, 3–15 (1999).
Gallop, M.A., Barrett, R.W., Dower, W.J., Fodor, S.P. & Gordon, E.M. Applications of combinatorial technologies to drug discovery. 1. Background and peptide combinatorial libraries. J. Med. Chem. 37, 1233–1251 (1994).
Gordon, E.M., Barrett, R.W., Dower, W.J., Fodor, S.P. & Gallop, M.A. Applications of combinatorial technologies to drug discovery. 2. Combinatorial organic synthesis, library screening strategies, and future directions. J. Med. Chem. 37, 1385–1401 (1994).
Woodbury Jr., C.P. & Venton, D.L. Methods of screening combinatorial libraries using immobilized or restrained receptors. J. Chromatogr. B Biomed. Sci. Appl. 725, 113–137 (1999).
Scott, J.K. & Smith, G.P. Searching for peptide ligands with an epitope library. Science 249, 386–390 (1990).
Barrett, R.W. et al. Selective enrichment and characterization of high affinity ligands from collections of random peptides on filamentous phage. Anal. Biochem. 204, 357–364 (1992).
Mondorf, K., Kaufman, D.B. & Carbonell, R.G. Screening of combinatorial peptide libraries: identification of ligands for affinity purification of proteins using a radiological approach. J. Pept. Res. 52, 526–536 (1998).
Teng, S.F., Sproule, K., Hussain, A. & Lowe, C.R. A strategy for the generation of biomimetic ligands for affinity chromatography. Combinatorial synthesis and biological evaluation of an IgG binding ligand. J. Mol. Recognit. 12, 67–75 (1999).
Burton, N.P. & Lowe, C.R. Design of novel affinity adsorbents for the purification of trypsin-like proteases. J. Mol. Recognit. 5, 55–68 (1992).
Sproule, K. et al. New strategy for the design of ligands for the purification of pharmaceutical proteins by affinity chromatography. J. Chromatogr. B Biomed. Sci. Appl. 740, 17–33 (2000).
Lowe, C.R. Combinatorial approaches to affinity chromatography. Curr. Opin. Chem. Biol. 5, 248–256 (2001).
Huang, P.Y. et al. Affinity purification of von Willebrand factor using ligands derived from peptide libraries. Bioorg. Med. Chem. 4, 699–708 (1996).
Kaufman, D.B. et al. Affinity purification of fibrinogen using a ligand from a peptide library. Biotechnol. Bioeng. 77, 278–289 (2002).
Oldenburg, K.R., Loganathan, D., Goldstein, I.J., Schultz, P.G. & Gallop, M.A. Peptide ligands for a sugar-binding protein isolated from a random peptide library. Proc. Natl. Acad. Sci. USA 89, 5393–5397 (1992).
Clonis, Y.D. Affinity chromatography matures as bioinformatic and combinatorial tools develop. J. Chromatogr. A 1101, 1–24 (2006).
Saraswat, L.D. et al. Affinity ligand selection from a library of small molecules: assay development, screening, and application. Biotechnol. Prog. 21, 300–308 (2005).
Paborsky, L.R., Dunn, K.E., Gibbs, C.S. & Dougherty, J.P. A nickel chelate microtiter plate assay for six histidine-containing proteins. Anal. Biochem. 234, 60–65 (1996).
Draveling, C., Ren, L., Haney, P., Zeisse, D. & Qoronfleh, M.W. SwellGel: an affinity chromatography technology for high-capacity and high-throughput purification of recombinant-tagged proteins. Protein Expr. Purif. 22, 359–366 (2001).
Mazza, C.B. et al. High-throughput screening and quantitative structure-efficacy relationship models of potential displacer molecules for ion-exchange systems. Biotechnol. Bioeng. 80, 60–72 (2002).
Rege, K., Hu, S., Moore, J.A., Dordick, J.S. & Cramer, S.M. Chemoenzymatic synthesis and high-throughput screening of an aminoglycoside-polyamine library: identification of high-affinity displacers and DNA-binding ligands. J. Am. Chem. Soc. 126, 12306–12315 (2004).
Rege, K., Ladiwala, A. & Cramer, S.M. Multidimensional high-throughput screening of displacers. Anal. Chem. 77, 6818–6827 (2005).
Rege, K., Ladiwala, A., Tugcu, N., Breneman, C.M. & Cramer, S.M. Parallel screening of selective and high-affinity displacers for proteins in ion-exchange systems. J. Chromatogr. A 1033, 19–28 (2004).
Rege, K., Tugcu, N. & Cramer, S.M. Predicting column performance in displacement chromatography from high throughput screening batch experiments. Sep. Sci. Technol. 38, 1499–1517 (2003).
Rege, K., Pepsin, M., Falcon, B., Steele, L. & Heng, M. High-throughput process development for recombinant protein purification. Biotechnol. Bioeng. 93, 618–630 (2006).
Coffman, J.L., Kramarczyk, J.F. & Kelley, B.D. High-throughput screening of chromatographic separations: I. Method development and column modeling. Biotechnol. Bioeng. 100, 605–618 (2008).
McNay, J.L. & Fernandez, E.J. Protein unfolding during reversed-phase chromatography: I. Effect of surface properties and duration of adsorption. Biotechnol. Bioeng. 76, 224–232 (2001).
McNay, J.L., O'Connell, J.P. & Fernandez, E.J. Protein unfolding during reversed-phase chromatography: II. Role of salt type and ionic strength. Biotechnol. Bioeng. 76, 233–240 (2001).
Bernard, A. & Payton, M. Selection of Escherichia coli expression systems. Curr. Protoc. Protein Sci. Ch. 5, Unit 5.2, 5.2.1–5.2.18 (2001).
Andrew, S.M., Titus, J.A. & Zumstein, L. Dialysis and concentration of protein solutions. Curr. Protoc. Cell Biol. Appendix 3, A.3C.1–A.3C.5 (2001).
Hagel, L. Gel-filtration chromatography. Curr. Protoc. Mol. Biol. Chapter 10, unit 10 19 (2001).
Author information
Authors and Affiliations
Contributions
K.R. and M.H. designed the work, K.R. carried out experiments, and K.R. and M.H. analyzed the data and wrote the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Rege, K., Heng, M. Miniaturized parallel screens to identify chromatographic steps required for recombinant protein purification. Nat Protoc 5, 408–417 (2010). https://doi.org/10.1038/nprot.2009.149
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.149
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.