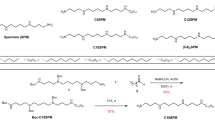
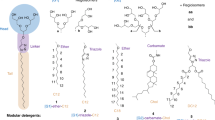
Abstract
For structural studies of integral membrane proteins, including their 3D crystallization, the judicious use of detergent for solubilization and purification is required. Detergent binding by the solubilized protein is an important parameter to determine the hydrodynamic properties in terms of size and aggregational (monomeric/oligo(proto)meric) state of the protein. Detergent binding can be measured by gel filtration chromatography under equilibrium conditions and after separation from mixed micelles of solubilized lipid and detergent. Using sarcoplasmic reticulum Ca2+-ATPase as an example, we demonstrate in this protocol complete procedures for measurement of detergent binding using (i) radiolabeled n-dodecyl-β-D-maltoside (DM) or (ii) from measurements of the increase in refractive index due to the presence of bound detergent on the protein. The latter measurement can also be performed by sedimentation velocity (SV) analysis in the analytical ultracentrifuge which in addition allows determination of the sedimentation coefficient. In combination with estimation of Stokes radius by gel filtration calibration, the molecular mass and asymmetry of the solubilized protein can be calculated. In the proposed protocols, the gel chromatographic procedures require 1 d; SV experiments are performed just after size exclusion. The whole time for these experiments is 24 h. Data analysis of analytical ultracentrifugation requires a couple of days.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kragh-Hansen, U., le Maire, M. & Møller, J.V. The mechanism of detergent solubilization of liposomes and protein-containing membranes. Biophys. J. 75, 2932–2946 (1998).
le Maire, M., Champeil, P. & Møller, J.V. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta. 1508, 86–111 (2000).
Møller, J.V. & le Maire, M. Detergent binding as a measure of hydrophobic surface area of integral membrane proteins. J. Biol. Chem. 268, 18659–18672 (1993).
Lund, S. et al. Detergent structure and associated lipid as determinants in the stabilization of solubilized Ca2+-ATPase from sarcoplasmic reticulum. J. Biol. Chem. 264, 4907–4915 (1989).
Simons, K., Helenius, A. & Garoff, H. Solubilization of the membrane proteins from Semliki Forest virus with Triton X100. J. Mol. Biol. 80, 119–133 (1973).
Makino, S., Reynolds, J.A. & Tanford, C. The binding of deoxycholate and Triton X-100 to proteins. J. Biol. Chem. 248, 4926–4932 (1973).
Eiselé, J.L. & Rosenbusch, J.P. Crystallization of porin using short chain phospholipids. J. Mol. Biol. 206, 209–212 (1989).
Reiss-Husson, F. & Picot, D. Crystallization of membrane proteins. In Crystallization of Nucleic Acids and Proteins: A Practical Approach, 2nd edn. (eds. Ducruix, A. & Giegé, R.) 246–268 (Oxford University Press, Oxford, UK, 1999).
Dahout-Gonzalez, C., Brandolin, G. & Pebay-Peyroula, E. Crystallization of the bovine ADP/ATP carrier is critically dependent upon the detergent-to-protein ratio. Acta Crystallogr. D Biol. Crystallogr. 59, 2353–2355 (2003).
Jidenko, M. et al. Crystallization of a mammalian membrane protein overexpressed in Saccharomyces cerevisiae . Proc. Natl. Acad. Sci. USA 102, 11687–11691 (2005).
Champeil, P., Menguy, T., Tribet, C., Popot, J.L. & le Maire, M. Interaction of amphipols with sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 275, 18623–18637 (2000).
Ebel, C., Møller, J.V. & le Maire, M. Analytical ultra centrifugation: membrane protein assemblies in the presence of detergent. In Biophysical Analysis of Membrane Proteins. Investigating Structure And Function (ed. Pebay-Peyroula, E.) 91–120 (Wiley, Weinheim, 2007).
Salvay, A.G., Santamaria, M., le Maire, M. & Ebel, C. Analytical ultracentrifugation sedimentation velocity for the characterization of detergent-solubilized membrane proteins Ca.-ATPase and ExbB. J. Biol. Phys. (in the press) doi: 10.1007/s10867-008-9058-3 (2008).
le Maire, M., Kwee, S., Andersen, J.P. & Møller, J.V. Mode of interaction of polyoxyethyleneglycol detergents with membrane proteins. Eur. J. Biochem. 129, 525–532 (1983).
le Maire, M., Aggerbeck, L.P., Monteilhet, C., Andersen, J.P. & Møller, J.V. The use of high-performance liquid chromatography for the determination of size and molecular weight of proteins: a caution and a list of membrane proteins suitable as standards. Anal. Biochem. 154, 525–535 (1986).
le Maire, M., Viel, A. & Møller, J.V. Size exclusion chromatography and universal calibration of gel columns. Anal. Biochem. 177, 50–56 (1989).
Boulanger, P. et al. Purification and structural and functional characterization of FhuA, a transporter of the Escherichia coli outer membrane. Biochemistry 35, 14216–14224 (1996).
Plancon, L. et al. Characterization of a high-affinity complex between the bacterial outer membrane protein FhuA and the phage T5 protein pb5. J. Mol. Biol. 318, 557–569 (2002).
Winstone, T.L. et al. Organic solvent extracted EmrE solubilized in dodecyl maltoside is monomeric and binds drug ligand. Biochem. Biophys. Res. Commun. 327, 437–445 (2005).
Ravaud, S. et al. The ABC transporter BmrA from Bacillus subtilis is a functional dimer when in a detergent-solubilized state. Biochem. J. 395, 345–353 (2006).
Urbani, A. & Warne, T. A colorimetric determination for glycosidic and bile salt-based detergents: applications in membrane protein research. Anal. Biochem. 336, 117–124 (2005).
Yernool, D., Boudker, O., Folta-Stogniew, E. & Gouaux, E. Trimeric subunit stoichiometry of the glutamate transporters from Bacillus caldotenax and Bacillus stearothermophilus . Biochemistry 42, 12981–12988 (2003).
Friesen, R.H., Knol, J. & Poolman, B. Quaternary structure of the lactose transport protein of streptococcus thermophilus in the detergent-solubilized and membrane-reconstituted state. J. Biol. Chem. 275, 40658 (2000).
Butler, P.J., Ubarretxena-Belandia, I., Warne, T. & Tate, C.G. The Escherichia coli multidrug transporter EmrE is a dimer in the detergent-solubilised state. J. Mol. Biol. 340, 797–808 (2004).
Bamber, L., Harding, M., Butler, P.J. & Kunji, E.R. Yeast mitochondrial ADP/ATP carriers are monomeric in detergents. Proc. Natl. Acad. Sci. USA 103, 16224–16229 (2006).
Eriks, L.R., Mayor, J.A. & Kaplan, R.S. A strategy for identification and quantification of detergents frequently used in the purification of membrane proteins. Anal. Biochem. 323, 234–241 (2003).
Deng, G., Chow, D. & Sanyal, G. Quantitative determination of saccharide surfactants in protein samples by liquid chromatography coupled to electrospray ionization mass spectrometry. Anal. Biochem. 289, 124–129 (2001).
Maezawa, S. et al. Determination of molecular weight of membrane proteins by the use of low-angle laser light scattering combined with high-performance gel chromatography in the presence of a non-ionic surfactant. Biochim. Biophys. Acta. 747, 291–297 (1983).
Hayashi, Y., Matsui, H. & Takagi, T. Membrane protein molecular weight determined by low-angle laser light-scattering photometry coupled with high-performance gel chromatography. Methods Enzymol. 172, 514–528 (1989).
Salvay, A.G. & Ebel, C. Analytical ultracentrifuge for the characterization of detergent in solution. Prog. Colloid Polym. Sci. 131, 74–82 (2006).
Karin, M., Liu, Z. & Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 9, 240–246 (1997).
Csúcs, G. & Ramsden, J.J. Solubilization of planar bilayers with detergent. Biochim. Biophys. Acta. 1369, 304–308 (1998).
Strop, P. & Brunger, A.T. Refractive index-based determination of detergent concentration and its application to the study of membrane proteins. Protein Sci. 14, 2207–2211 (2005).
Tanford, C., Nozaki, Y., Reynolds, J.A. & Makino, S. Molecular characterization of proteins in detergent solutions. Biochemistry 13, 2369–2376 (1974).
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 78, 1606–1619 (2000).
Edelstein, S.J. & Schachman, H.K. Measurement of partial specific volume by sedimentation equilibrium in H2O-D2O solutions. Methods Enzymol. 27, 82–98 (1973).
Reynolds, J.A. & Tanford, C. Determination of molecular weight of the protein moiety in protein-detergent complexes without direct knowledge of detergent binding. Proc. Natl. Acad. Sci. USA 73, 4467–4470 (1976).
Burgess, N.K., Stanley, A.M. & Fleming, K.G. Determination of membrane protein molecular weights and association equilibrium constants using sedimentation equilibrium and sedimentation velocity. Methods Cell Biol. 84, 181–211 (2008).
Butler, P.J.P. & Tate, C.G. Correcting the buoyancy of macromolecules: density increments and apparent partial specific volumes with particular reference to the study of membrane proteins. In Modern Analytical Ultracentrifugation: Techniques and Methods (eds. Scott, D.J., Harding, S.E. & Rowe, A.J.) 133–151 (The Royal Society of Chemistry, Cambridge, 2006).
Kragh-Hansen, U., le Maire, M., Noel, J.P., Gulik-Krzywicki, T. & Møller, J.V. Transitional steps in the solubilization of protein-containing membranes and liposomes by nonionic detergent. Biochemistry 32, 1648–1656 (1993).
Georgin, D., le Maire, M. & Noël, J.P. Syntheses of [14C]-detergents. J. Labelled Comp. Radiopharm. 44, 575–585 (2001).
de Foresta, B. et al. Membrane solubilization by detergent: use of brominated phospholipids to evaluate the detergent-induced changes in Ca2+-ATPase/lipid interaction. Biochemistry 28, 2558–2567 (1989).
le Maire, M., Chabaud, R. & Hervé, G. Laboratory Guide to Biochemistry, Enzymology, and Protein Physical Chemistry (Plenum Publishing Corporation, New York, 1991).
Peterson, G.L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal. Biochem. 100, 201–220 (1979).
Kaplan, R.S. & Pedersen, P.L. Sensitive protein assay in presence of high levels of lipid. Methods Enzymol. 172, 393–399 (1989).
Bartlett, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 234, 466–468 (1959).
Josse, D. et al. Oligomeric states of the detergent-solubilized human serum paraoxonase (PON1). J. Biol. Chem. 277, 33386–33397 (2002).
Cohen, E. et al. Purification of Na+,K+-ATPase expressed in Pichia pastoris reveals an essential role of phospholipid-protein interactions. J. Biol. Chem. 280, 16610–16618 (2005).
Acknowledgements
This study was funded in part by grants from the Commissariat à l'Energie Atomique program Signalisation et transport membranaires (to M.l.M. and C.E.) and in part by an Agence Nationale de la Recherche grant (ANR-06-BLAN-0239-01) (to M.l.M.), by the Danish Medical Research Council, Aarhus University Research Foundation, and The Vilhelm Pedersen Foundation (to J.V.M.) and a Ph.D. stipend to C.O. from the Medical Faculty, Aarhus University. This protocol was improved over the years, in particular, thanks to the biennial EMBO Practical Course 'Current Methods in Membrane Protein Research' at The European Molecular Biology Laboratory, Heidelberg, Germany, organized in part by the late Matti Saraste to whom we would like to dedicate this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
le Maire, M., Arnou, B., Olesen, C. et al. Gel chromatography and analytical ultracentrifugation to determine the extent of detergent binding and aggregation, and Stokes radius of membrane proteins using sarcoplasmic reticulum Ca2+–ATPase as an example. Nat Protoc 3, 1782–1795 (2008). https://doi.org/10.1038/nprot.2008.177
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.177
This article is cited by
-
Sedimentation Velocity Analytical Ultracentrifugation Analysis of Marketed Rituximab Drug Product Size Distribution
Pharmaceutical Research (2020)
-
Quantification of Detergents Complexed with Membrane Proteins
Scientific Reports (2017)
-
Characterising protein/detergent complexes by triple-detection size-exclusion chromatography
Biological Procedures Online (2016)
-
SERCA mutant E309Q binds two Ca2+ions but adopts a catalytically incompetent conformation
The EMBO Journal (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.