Abstract
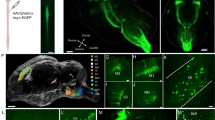
We provide a protocol that describes an explant system that allows the dynamics of motor axons to be imaged. This method is based on nerve–muscle explants prepared from the triangularis sterni muscle of mice, a thin muscle that covers the inside of the thorax. These explants, which can be maintained alive for several hours, contain long stretches of peripheral motor axons including their terminal arborizations and neuromuscular junctions. Explants can be prepared from transgenic mouse lines that express fluorescent proteins in neurons or glial cells, which enables direct visualization of their cellular and subcellular morphology by fluorescence microscopy. Time-lapse imaging then provides a convenient and reliable approach to follow the dynamic behavior of motor axons, their surrounding glial cells and their intracellular organelles with high temporal and spatial resolution. Triangularis sterni explants can be prepared in 15 min, imaged ex vivo for several hours and processed for immunohistochemistry in about 2 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McArdle, J.J. et al. Advantages of the triangularis sterni muscle of the mouse for investigations of synaptic phenomena. J. Neurosci. Methods 4, 109–115 (1981).
Brigant, J.L. & Mallart, A. Presynaptic currents in mouse motor endings. J. Physiol. 333, 619–636 (1982).
Angaut-Petit, D. & Mallart, A. Electrical activity of mouse motor endings during muscle reinervation. Neurosci. 16, 1047–1056 (1985).
Angaut-Petit, D. et al. Electrophysiological and morphological studies of a motor nerve in 'motor endplate disease' of the mouse. Proc. Soc. Lond. B Biol. Sci. 215, 117–125 (1982).
Bournaud, R. & Mallart, A. Potassium channel blockers and impulse propagation in murine motor endplate disease. Muscle Nerve 10, 1–5 (1987).
Bishop, D.L., Misgeld, T., Walsh, M.K., Gan, W.B. & Lichtman, J.W. Axon branch removal at developing synapses by axosome shedding. Neuron 44, 651–661 (2004).
Misgeld, T., Kerschensteiner, M., Bareyre, F.M., Burgess, R.W. & Lichtman, J.W. Imaging axonal transport of mitochondria in vivo. Nat. Methods 4, 559–561 (2007).
Duron, B., Jung-Caillol, M.C. & Marlot, D. Myelinated nerve fiber supply and muscle spindles in the respiratory muscles of cat: quantitative study. Anat. Embryol. (Berl) 152, 171–192 (1978).
Keller-Peck, C.R. et al. Asynchronous synapse elimination in neonatal motor units: studies using GFP transgenic mice. Neuron 31, 381–394 (2001).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007).
Parson, S.H. et al. Axotomy-dependent and -independent synapse elimination in organ cultures of Wld(s) mutant mouse skeletal muscle. J. Neurosci. Res. 76, 64–75 (2004).
Walsh, M.K. & Lichtman, J.W. Imaging the development of the neuromuscular junction. In Imaging in Neuroscience and Development (eds. Yuste, R. & Konnerth, A.) 215–223 (2005).
Walsh, M.K. & Lichtman, J.W. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron 37, 67–73 (2003).
Schaefer, A.M., Sanes, J.R. & Lichtman, J.W. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J. Comp. Neurol. 490, 209–219 (2005).
Sanes, J.R. & Lichtman, J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 (1999).
Lichtman, J.W. & Sanes, J.R. Watching the neuromuscular junction. J. Neurocytol. 32, 767–775 (2003).
Lichtman, J.W. & Colman, H. Synapse elimination and indelible memory. Neuron 25, 269–278 (2000).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
De Paola, V., Arber, S. & Caroni, P. AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nat. Neurosci. 6, 491–500 (2003).
Misgeld, T. & Kerschensteiner, M. In vivo imaging of the diseased nervous system. Nat. Rev. Neurosci. 7, 449–463 (2006).
Zuo, Y. et al. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. J. Neurosci. 24, 10999–11009 (2004).
Noguchi, S., Wakabayashi-Takai, E., Sasaoka, T. & Ozawa, E. Analysis of the spatial, temporal and tissue-specific transcription of gamma-sarcoglycan gene using a transgenic mouse. FEBS Lett. 495, 77–81 (2001).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell Biol. 20, 4106–4114 (2000).
Marques, M.J., Conchello, J.A. & Lichtman, J.W. From plaque to pretzel: fold formation and acetylcholine receptor loss at the developing neuromuscular junction. J. Neurosci. 20, 3663–3675 (2000).
Parson, S.H., Mackintosh, C.L. & Ribchester, R.R. Elimination of motor nerve terminals in neonatal mice expressing a gene for slow Wallerian degeneration (C57Bl/Wlds). Eur. J. Neurosci. 9, 1586–1592 (1997).
Wyatt, R.M. & Balice-Gordon, R.J. Heterogeneity in synaptic vesicle release at neuromuscular synapses of mice expressing synaptopHluorin. J. Neurosci. 28, 325–335 (2008).
Lichtman, J.W., Magrassi, L. & Purves, D. Visualization of neuromuscular junctions over periods of several months in living mice. J. Neurosci. 7, 1215–1222 (1987).
Acknowledgements
We thank A. Mauermayer (Technical University, Munich) for help with the photographs, and J.R. Sanes (Harvard University) for first pointing us to J.J. McArdle's work on the triangularis sterni muscle, which is the basis for the explant system described here. Work in M.K.'s laboratory is financed through grants from the Deutsche Forschungsgemeinschaft (DFG; Emmy-Noether Program and SFB 571), the Hertie-Foundation and the 'Verein Therapieforschung für MS-Kranke e.V.'. M.R. is supported by a predoctoral fellowship of the International Research Training Group 1373 'Brain Signalling: From Neurons to Circuits'. T.M. is supported by the Institute of Advanced Studies (Technical University, Munich), by the Alexander-von-Humboldt-Foundation, the DFG, the Hertie-Foundation and the Center of Integrated Protein Science, Munich. Work on MitoMice was also supported by a grant from the Dana-Foundation to T.M. and M.K.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kerschensteiner, M., Reuter, M., Lichtman, J. et al. Ex vivo imaging of motor axon dynamics in murine triangularis sterni explants. Nat Protoc 3, 1645–1653 (2008). https://doi.org/10.1038/nprot.2008.160
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.160
This article is cited by
-
Congenic expression of poly-GA but not poly-PR in mice triggers selective neuron loss and interferon responses found in C9orf72 ALS
Acta Neuropathologica (2020)
-
Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity
Nature Neuroscience (2019)
-
Differences in the constituent fiber types contribute to the intermuscular variation in the timing of the developmental synapse elimination
Scientific Reports (2019)
-
FE65 and FE65L1 share common synaptic functions and genetically interact with the APP family in neuromuscular junction formation
Scientific Reports (2016)
-
Mitochondrial redox and pH signaling occurs in axonal and synaptic organelle clusters
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.