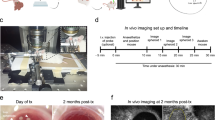
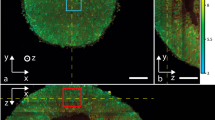
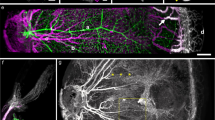
Abstract
There is clearly a demand for an experimental platform that enables cell biology to be studied in intact vascularized and innervated tissue in vivo. This platform should allow observations of cells noninvasively and longitudinally at single-cell resolution. For this purpose, we use the anterior chamber of the mouse eye in combination with laser scanning microscopy (LSM). Tissue transplanted to the anterior chamber of the eye is rapidly vascularized, innervated and regains function. After transplantation, LSM through the cornea allows repetitive and noninvasive in vivo imaging at cellular resolution. Morphology, vascularization, cell function and cell survival are monitored longitudinally using fluorescent proteins and dyes. We have used this system to study pancreatic islets, but the platform can easily be adapted for studying a variety of tissues and additional biological parameters. Transplantation to the anterior chamber of the eye takes 25 min, and in vivo imaging 1–5 h, depending on the features monitored.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Koo, V., Hamilton, P.W. & Williamson, K. Non-invasive in vivo imaging in small animal research. Cell. Oncol. 28, 127–139 (2006).
Massoud, T.F. & Gambhir, S.S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 17, 545–580 (2003).
Dunn, K.W. & Sutton, T.A. Functional studies in living animals using multiphoton microscopy. ILAR J. 49, 66–77 (2008).
Handbook of Biological Confocal Microscopy 3rd edn. (ed. Pawley, J.B.) (Springer, New York, 2006).
Adeghate, E., Ponery, A.S., Ahmed, I. & Donath, T. Comparative morphology and biochemistry of pancreatic tissue fragments transplanted into the anterior eye chamber and subcutaneous regions of the rat. Eur. J. Morphol. 39, 257–268 (2001).
Katoh, N. et al. Target-specific innervation by autonomic and sensory nerve fibers in hairy fetal skin transplanted into the anterior eye chamber of adult rat. Cell Tissue Res. 266, 259–263 (1991).
Olson, L. & Seiger, A. Beating intraocular hearts: light-controlled rate by autonomic innervation from host iris. J. Neurobiol. 7, 193–203 (1976).
Wu, W., Scott, D.E. & Reiter, R.J. Transplantation of the mammalian pineal gland: studies of survival, revascularization, reinnervation, and recovery of function. Exp. Neurol. 122, 88–99 (1993).
Hoffer, B., Seiger, A., Ljungberg, T. & Olson, L. Electrophysiological and cytological studies of brain homografts in the anterior chamber of the eye: maturation of cerebellar cortex in oculo. Brain Res. 79, 165–184 (1974).
Niederkorn, J.Y. Immune privilege in the anterior chamber of the eye. Crit. Rev. Immunol. 22, 13–46 (2002).
Adeghate, E. Pancreatic tissue grafts are reinnervated by neuro-peptidergic and cholinergic nerves within five days of transplantation. Transpl. Immunol. 10, 73–80 (2002).
Adeghate, E. Host-graft circulation and vascular morphology in pancreatic tissue transplants in rats. Anat. Rec. 251, 448–459 (1998).
Speier, S. et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat. Med. 14, 574–578 (2008).
Bertera, S. et al. Body window-enabled in vivo multicolor imaging of transplanted mouse islets expressing an insulin-Timer fusion protein. BioTechniques 35, 718–722 (2003).
Martinic, M.M. & von Herrath, M.G. Real-time imaging of the pancreas during development of diabetes. Immunol. Rev. 221, 200–213 (2008).
Zhuravleva, Z.N., Bragin, A.G. & Vinogradova, O.S. Organization of the nervous tissue (hippocampus and septum) developing in the anterior eye chamber. I. General characteristic and non-neural elements. J. Hirnforsch. 25, 313–330 (1984).
Adeghate, E. & Donath, T. Distribution of neuropeptide Y and vasoactive intestinal polypeptide immunoreactive nerves in normal and transplanted pancreatic tissue. Peptides 11, 1087–1092 (1990).
Boutet de Monvel, J., Le Calvez, S. & Ulfendahl, M. Image restoration for confocal microscopy: improving the limits of deconvolution, with application to the visualization of the mammalian hearing organ. Biophys. J. 80, 2455–2470 (2001).
Köhler, M. et al. On-line monitoring of apoptosis in insulin-secreting cells. Diabetes 52, 2943–2950 (2003).
Berney, T. et al. Endotoxin-mediated delayed islet graft function is associated with increased intra-islet cytokine production and islet cell apoptosis. Transplantation 71, 125–132 (2001).
Nyqvist, D., Köhler, M., Wahlstedt, H. & Berggren, P.O. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes 54, 2287–2293 (2005).
Bernd, A.S., Aihara, M., Lindsey, J.D. & Weinreb, R.N. Influence of molecular weight on intracameral dextran movement to the posterior segment of the mouse eye. Invest. Ophthalmol. Vis. Sci. 45, 480–484 (2004).
Aynsley-Green, A., Biebuyck, J.F. & Alberti, K.G. Anaesthesia and insulin secretion: the effects of diethyl ether, halothane, pentobarbitone sodium and ketamine hydrochloride on intravenous glucose tolerance and insulin secretion in the rat. Diabetologia 9, 274–281 (1973).
Brown, E.T., Umino, Y., Loi, T., Solessio, E. & Barlow, R. Anesthesia can cause sustained hyperglycemia in C57/BL6J mice. Vis. Neurosci. 22, 615–618 (2005).
Desborough, J.P., Jones, P.M., Persaud, S.J., Landon, M.J. & Howell, S.L. Isoflurane inhibits insulin secretion from isolated rat pancreatic islets of Langerhans. Br. J. Anaesth. 71, 873–876 (1993).
Acknowledgements
This study was supported by grants DK-58508 and DK-075487 (to A.C.) from the US National Institutes of Health, Juvenile Diabetes Research Foundation International grant 3-2007-73 (to S.S.) and 4-2004-361, the Swedish Research Council, the Novo Nordisk Foundation, Karolinska Institutet, the Swedish Diabetes Association, The Family Knut and Alice Wallberg Foundation, Eurodia (FP6-518153), European Foundation for the Study of Diabetes, the EFSD/Lilly Research Program, Berth von Kantzow's Foundation, the Family Erling-Persson Foundation and the Diabetes Research Institute Foundation (Hollywood, FL).
Author information
Authors and Affiliations
Contributions
The first two authors contributed equally to this work.
Corresponding author
Rights and permissions
About this article
Cite this article
Speier, S., Nyqvist, D., Köhler, M. et al. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat Protoc 3, 1278–1286 (2008). https://doi.org/10.1038/nprot.2008.118
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.118
This article is cited by
-
Intraocular liver spheroids for non-invasive high-resolution in vivo monitoring of liver cell function
Nature Communications (2024)
-
Improved in vivo imaging method for individual islets across the mouse pancreas reveals a heterogeneous insulin secretion response to glucose
Scientific Reports (2021)
-
Topologically selective islet vulnerability and self-sustained downregulation of markers for β-cell maturity in streptozotocin-induced diabetes
Communications Biology (2020)
-
Noninvasive intravital high-resolution imaging of pancreatic neuroendocrine tumours
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.