Abstract
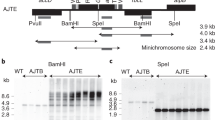
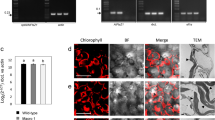
Several major costs associated with the production of biopharmaceuticals or vaccines in fermentation-based systems could be minimized by using plant chloroplasts as bioreactors, which facilitates rapid scale-up. Oral delivery of chloroplast-derived therapeutic proteins through plant cells eliminates expensive purification steps, low temperature storage, transportation and sterile injections for their delivery. Chloroplast transformation technology (CTT) has also been successfully used to engineer valuable agronomic traits and for the production of industrial enzymes and biomaterials. Here, we provide a detailed protocol for the construction of chloroplast expression and integration vectors, selection and regeneration of transformants, evaluation of transgene integration and inheritance, confirmation of transgene expression and extraction, and quantitation and purification of foreign proteins. Integration of appropriate transgenes into chloroplast genomes and the resulting high levels of functional protein expression can be achieved in ∼6 months in lettuce and tobacco. CTT is eco-friendly because transgenes are maternally inherited in most crop plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Koya, V., Moayeri, M., Leppla, S.H. & Daniell, H. Plant-based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect. Immun. 73, 8266–8274 (2005).
Daniell, H. Molecular strategies for gene containment in transgenic crops. Nat. Biotechnol. 20, 581–586 (2002).
Hagemann, R. The sexual inheritance of plant organelles. In Molecular Biology and Biotechnology of Plant Organelles (eds. Daniell, H. & Chase, C.) 93–113 (Springer Publishers, Dordrecht, The Netherlands, 2004).
Daniell, H. Transgene containment by maternal inheritance: effective or elusive? Proc. Natl. Acad. Sci. USA 104, 6879–6880 (2007).
Ruiz, O.N. & Daniell, H. Engineering cytoplasmic male sterility via the chloroplast genome by expression of {beta}-ketothiolase. Plant Physiol. 138, 1232–1246 (2005).
Arlen, P.A. et al. Field production and functional evaluation of chloroplast-derived interferon alpha2b. Plant Biotechnol. J 5, 511–525 (2007).
DeCosa, B., Moar, W., Lee, S.B., Miller, M. & Daniell, H. Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat. Biotechnol. 19, 71–74 (2001).
Grevich, J.J. & Daniell, H. Chloroplast genetic engineering: recent advances and future perspectives. Crit. Rev. Plant Sci. 24, 83–107 (2005).
Daniell, H., Kumar, S. & Dufourmantel, N. Breakthrough in chloroplast genetic engineering of agronomically important crops. Trends Biotechnol. 23, 238–245 (2005).
Chilton, M.M. & Que, Q. Targeted integration of T-DNA into the tobacco genome at double-stranded breaks: new insights on the mechanism of T-DNA integration. Plant Physiol. 133, 956–965 (2003).
Dhingra, A., Portis, A.R. Jr. & Daniell, H. Enhanced translation of a chloroplast-expressed RbcS gene restores small subunit levels and photosynthesis in nuclear RbcS antisense plants. Proc. Natl. Acad. Sci. USA 101, 6315–6320 (2004).
Lee, S.B. et al. Accumulation of trehalose within transgenic chloroplasts confers drought tolerance. Mol. Breed. 11, 1–13 (2003).
Quesada-Vargas, T., Ruiz, O.N. & Daniell, H. Characterization of heterologous multigene operons in transgenic chloroplasts: transcription, processing, and translation. Plant Physiol. 138, 1746–1762 (2005).
Staub, J.M. et al. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat. Biotechnol. 18, 333–338 (2000).
Leelavathi, S. & Reddy, V.S. Chloroplast expression of his-tagged GUS-fusions: a general strategy to overproduce and purify foreign proteins using transplastomic plants as bioreactors. Mol. Breed. 11, 49–58 (2003).
Ruhlman, T., Ahangari, R., Devine, A., Samsam, M. & Daniell, H. Oral delivery of chloroplast derived cholera toxin B-proinsulin protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol. J. 5, 495–510 (2007).
Daniell, H., Lee, S.B., Panchal, T. & Wiebe, P.O. Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J. Mol. Biol. 311, 1001–1009 (2001).
Chebolu, S. & Daniell, H. Stable expression of Gal/GalNAc lectin of Entamoeba histolytica in transgenic chloroplasts and immunogenicity in mice towards vaccine development for amoebiasis. Plant Biotechnol. J. 5, 230–239 (2007).
Birch-Machin, I., Newell, C.A., Hibberd, J.M. & Gray, J.C. Accumulation of rotavirus VP6 protein in chloroplasts of transplastomic tobacco is limited by protein stability. Plant Biotechnol. J. 2, 261–270 (2004).
Glenz, K. et al. Production of a recombinant bacterial lipoprotein in higher plant chloroplasts. Nat. Biotechnol. 24, 76–77 (2006).
Fischer, R. & Emans, N. Molecular farming of pharmaceutical proteins. Transgenic Res. 9, 279–299 (2000).
Cramer, C.L., Boothe, J.G. & Oishi, K.K. Transgenic plants for therapeutic proteins: linking upstream and downstream strategies. Curr. Top. Microbiol. Immunol. 240, 95–118 (1999).
Sidorov, V.A. et al. Technical advance: stable chloroplast transformation in potato: use of green fluorescent protein as a plastid marker. Plant J. 19, 209–216 (1999).
Ruf, S., Hermann, M., Berger, I.J., Carrer, H. & Bock, R. Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat. Biotechnol. 19, 870–875 (2001).
Daniell, H., Vivekananda, J., Nielsen, B.L., Ye, G.N. & Tewari, K.K. Transient foreign gene expression in chloroplasts of cultured tobacco cells after biolistic delivery of chloroplast vectors. Proc. Natl. Acad. Sci. USA 87, 88–92 (1990).
Kunnimalaiyaan, M. & Nielsen, B.L. Fine mapping of replication origins (ori A and ori B) in Nicotiana tabacum chloroplast DNA. Nucleic Acids Res. 25, 3681–3686 (1997).
Allison, L.A., Simon, L.D. & Maliga, P. Deletion of rpoB reveals a second distinct transcription system in plastids of higher plants. EMBO J. 15, 2802–2809 (1996).
Ruiz, O.N., Hussein, H.S., Terry, N. & Daniell, H. Phytoremediation of organomercurial compounds via chloroplast genetic engineering. Plant Physiol. 132, 1344–1352 (2003).
Magee, A. et al. T7 RNA polymerase-directed expression of an antibody fragment transgene in plastids causes a semi-lethal pale-green seedling phenotype. Transgenic Res. 13, 325–337 (2004).
Muhlbauer, S.K. & Koop, H.U. External control of transgene expression in tobacco plastids using the bacterial lac repressor. Plant J. 43, 941–946 (2005).
Daniell, H., Carmona-Sanchez, O. & Burns, B. Chloroplast derived antibodies, biopharmaceuticals and edible vaccines. In Molecular Farming (eds. Schillberg, S. & Wiley, V.C.H.) 113–133 (Verlag publishers, Germany, 2004).
Daniell, H. & McFadden, B.A Uptake and expression of bacterial and cyanobacterial genes by isolated cucumber etioplasts. Proc. Natl. Acad. Sci. USA 84, 6349–6353 (1987).
Boynton, J.E. et al. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 240, 1534–1538 (1988).
Golds, T., Maliga, P. & Koop, H.U. Stable plastid transformation in PEG-treated protoplasts of Nicotiana tabacum. Nat. Biotechnol. 11, 95–97 (1993).
O'Neill, C., Horvath, G.V., Horvath, E., Dix, P.J. & Medgyesy, P. Chloroplast transformation in plants: polyethylene glycol (PEG) treatment of protoplast is an alternative to biolistic delivery systems. Plant J. 3, 729–738 (1993).
Svab, Z. & Maliga, P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc. Natl. Acad. Sci. USA 90, 913–917 (1993).
Kumar, S., Dhingra, A. & Daniell, H. Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol. 136, 2843–2854 (2004).
Brosch, M., Krause, K., Falk, J. & Krupinska, K. Analysis of gene expression in amyloplasts of potato tubers. Planta 227, 91–99 (2007).
Watson, J., Koya, V., Leppla, S.H. & Daniell, H. Expression of Bacillus anthracis protective antigen in transgenic chloroplasts of tobacco, a non-food/feed crop. Vaccine 22, 4374–4384 (2004).
Tregoning, J.S. et al. Expression of tetanus toxin Fragment C in tobacco chloroplasts. Nucleic Acids Res. 31, 1174–1179 (2003).
Tregoning, J.S. et al. Protection against tetanus toxin using a plant-based vaccine. Eur. J. Immunol. 35, 1320–1326 (2005).
Molina, A., Hervás-Stubbs, S., Daniell, H., Mingo-Castel, A.M. & Veramendi, J. High-yield expression of a viral peptide animal vaccine in transgenic tobacco chloroplasts. Plant Biotechnol. J. 2, 141–153 (2004).
Molina, A., Veramendi, J. & Hervás-Stubbs, S. Induction of neutralizing antibodies by a tobacco chloroplast-derived vaccine based on a B cell epitope from canine parvovirus. Virology 342, 266–275 (2005).
Fernández-San Millán, A., Mingo-Castel, A., Miller, M. & Daniell, H. A chloroplast transgenic approach to hyper-express and purify Human Serum Albumin, a protein highly susceptible to proteolytic degradation. Plant Biotechnol. J. 1, 71–79 (2003).
DeGray, G., Rajasekaran, K., Smith, F., Sanford, J. & Daniell, H. Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol. 127, 852–862 (2001).
Kamarajugadda, S. & Daniell, H. Chloroplast-derived anthrax and other vaccine antigens: their immunogenic and immunoprotective properties. Expert Rev. Vaccines 5, 839–849 (2006).
Dufourmantel, N. et al. Generation of fertile transplastomic soybean. Plant Mol. Biol. 55, 479–489 (2004).
Kanamoto, H. et al. Efficient and stable transformation of Lactuca sativa L. cv. Cisco (lettuce) plastids. Transgenic Res. 15, 205–217 (2006).
Daniell, H., Chebolu, S., Kumar, S., Singleton, M. & Falconer, R. Chloroplast-derived vaccine antigens and other therapeutic proteins. Vaccine 23, 1779–1783 (2005).
Daniell, H. Production of biopharmaceuticals and vaccines in plants via the chloroplast genome. Biotechnol. J. 1, 1071–1079 (2006).
Leelavathi, S., Gupta, N., Maiti, S., Ghosh, A. & Reddy, V.S. Overproduction of an alkali- and thermo-stable xylanase in tobacco chloroplasts and efficient recovery of the enzyme. Mol. Breed. 11, 59–67 (2003).
Viitanen, P.V. et al. Metabolic engineering of the chloroplast genome using the Escherichia coli ubiC gene reveals that chorismate is a readily abundant plant precursor for p-hydroxybenzoic acid biosynthesis. Plant Physiol. 136, 4048–4060 (2004).
Molecular Cloning: A Laboratory Manual (eds. Sambrook, J. & Russell, D.W.) (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York 2001).
Costa, G.L. & Weiner, M.P. Bidirectional and directional cloning of PCR products. In PCR Primer 2nd edn. (eds. Dieffenbach C.W. and Dveksler G.S.) (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2003).
Lutz, A.L., Svab, Z. & Maliga, P. Construction of marker-free transplastomic tobacco using the Cre-loxP site-specific recombination system. Nat. Protoc. 1, 1–11 (2006).
Daniell, H., Ruiz, O.N. & Dhingra, A. Chloroplast genetic engineering to improve agronomic traits. Methods Mol. Biol. 286, 111–137 (2005).
Nugent, G.D., Coyne, S., Nguyen, T.T., Kavanagh, T.T. & Dix, P.J. Nuclear and plastid transformation of Brassica oleracea var. botrytis (cauliflower) using PEG-mediated uptake of DNA into protoplasts. Plant Sci. 170, 135–142 (2006).
Kumar, S., Dhingra, A. & Daniell, H. Stable transformation of the cotton plastid genome and maternal inheritance of transgenes. Plant Mol. Biol. 56, 203–216 (2004).
Lelivelt, C. et al. Stable plastid transformation in lettuce (Lactuca sativa L.). Plant Mol. Biol. 58, 763–774 (2005).
Hou, B.K. et al. Chloroplast transformation in oilseed rape. Transgenic Res. 12, 111–114 (2003).
Zubko, M., Zubko, E., Zuilen, K., Meyer, P. & Day, A. Stable transformation of petunia plastids. Transgenic Res. 13, 523–530 (2004).
Okumura, S. et al. Transformation of poplar (Populus alba) plastids and expression of foreign proteins in tree chloroplasts. Transgenic Res. 15, 637–646 (2006).
Nguyen, T.T., Nugent, G., Cardi, T. & Dix, P.J. Generation of homoplasmic plastid transformants of a commercial cultivar of potato (Solanum tuberosum L.). Plant Sci. 168, 1495–1500 (2005).
Lee, S.M. et al. Plastid transformation in the monocotyledonous cereal crop, rice (Oryza sativa) and transmission of transgenes to their progeny. Mol. Cells 21, 401–410 (2006).
Daniell, H., Datta, R., Varma, S., Gray, S. & Lee, S.B. Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat. Biotechnol. 16, 345–348 (1998).
McBride, K.E. et al. Amplification of a chimeric Bacillus gene in chloroplasts leads to an extraordinary level of an insecticidal protein in tobacco. Biotechnology 13, 362–365 (1995).
Kota, M. et al. Overexpression of the Bacillus thuringiensis (Bt) Cry2Aa2 protein in chloroplasts confers resistance to plants against susceptible and Bt-resistant insects. Proc. Natl. Acad. Sci. USA 96, 1840–1845 (1999).
Dufourmantel, N. et al. Generation and analysis of soybean plastid transformants expressing Bacillus thuringiensis Cry1Ab protoxin. Plant Mol. Biol. 58, 659–668 (2005).
Chakrabarti, S.K., Lutz, K.A., Lertwiriyawong, B., Svab, Z. & Maliga, P. Expression of the cry9Aa2 B.t. gene in tobacco chloroplasts confers resistance to potato tuber moth. Transgenic Res. 15, 481–488 (2006).
Iamtham, S. & Day, A. Removal of antibiotic resistance genes from transgenic tobacco plastids. Nat. Biotechnol. 18, 1172–1176 (2000).
Guda, C., Lee, S-B. & Daniell, H. Stable expression of a biodegradable protein-based polymer in tobacco chloroplasts. Plant Cell Rep. 19, 257–262 (2000).
Lössl, A., Eibl, C., Harloff, H.J., Jung, C. & Koop, H.U. Polyester synthesis in transplastomic tobacco (Nicotiana tabacum L.): significant contents of polyhydroxybutyrate are associated with growth reduction. Plant Cell Rep. 21, 891–899 (2003).
Zhang, X.H., Brotherton, J.E., Widholm, J.M. & Portis, A.R. Targeting a nuclear anthranilate synthase alpha-subunit gene to the tobacco plastid genome results in enhanced tryptophan biosynthesis: return of a gene to its pre-endosymbiotic origin. Plant Physiol. 127, 131–141 (2001).
Roh, K.H. et al. Accumulation of sweet protein monellin is regulated by the psbA 5′UTR in tobacco chloroplasts. J. Plant Biol. 49, 34–43 (2006).
Acknowledgements
The results reported in this article were supported in part by grants from United States Department of Agriculture 3611-21000-017-00D and National Institutes of Health R01 GM 63879 to H.D. The authors are grateful to Drs. Philip Arlen and Dolendro Singh for critically reading this article and Dr. Arlen for redrawing Figure 7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, D., Samson, N., Koya, V. et al. A protocol for expression of foreign genes in chloroplasts. Nat Protoc 3, 739–758 (2008). https://doi.org/10.1038/nprot.2007.522
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.522
This article is cited by
-
Chloroplast transformation in new cultivars of tomato through particle bombardment
3 Biotech (2024)
-
Carotenoid Pathway Engineering in Tobacco Chloroplast Using a Synthetic Operon
Molecular Biotechnology (2023)
-
Plastid engineering using episomal DNA
Plant Cell Reports (2023)
-
Enterohemorrhagic Escherichia coli O157:H7 antigens produced in transgenic lettuce effective as an oral vaccine in mice
Theoretical and Applied Genetics (2023)
-
A new prokaryotic expression vector for the expression of antimicrobial peptide abaecin using SUMO fusion tag
BMC Biotechnology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.