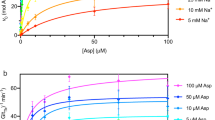
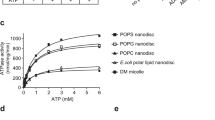
Abstract
In this protocol, we describe a procedure for incorporating ATP-binding cassette (ABC) transporters into large unilamellar vesicles (LUVs) and assays to determine ligand binding and solute translocation by these membrane-reconstituted systems. The reconstitution technique as described has been optimized for ABC transporters but can be readily adapted for other types of transport systems. Purified transporters are inserted into detergent-destabilized preformed liposomes and detergent is subsequently removed by adsorption onto polystyrene beads. Next, Mg-ATP or an ATP-regenerating system is incorporated into the vesicle lumen by one or more cycles of freezing-thawing, followed by extrusion through polycarbonate filters to obtain unilamellar vesicles. Binding and translocation of substrates are measured using isotope-labeled ligands and rapid filtration to separate the proteoliposomes from the surrounding medium. Quantitative information is obtained about dissociation constants (Kd) for ligand binding, number of binding-sites, transport affinities (Km), rates of transport, and the activities of transporter molecules with opposite orientations in the membrane. The full protocol can be completed within 4–5 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rigaud, J.L., Pitard, B. & Levy, D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim. Biophys. Acta 1231, 223–246 (1995).
Rigaud, J.L. & Lévy, D. Reconstitution of membrane proteins into liposomes. Methods Enzymol. 372, 65–86 (2003).
Holloway, P.W. A simple procedure for removal of Triton X-100 from protein samples. Anal. Biochem. 53, 304–308 (1973).
Poolman, B. et al. Functional analysis of detergent-solubilized and membrane-reconstituted ATP-binding cassette transporters. Methods Enzymol. 400, 429–459 (2005).
Almog, S. et al. States of aggregation and phase transformations in mixtures of phosphatidylcholine and octyl glucoside. Biochemistry 29, 4582–4592 (1990).
Knol, J. et al. Unidirectional reconstitution into detergent-destabilized liposomes of the purified lactose transport system of Streptococcus thermophilus. J. Biol. Chem. 271, 15358–15366 (1996).
Knol, J., Sjollema, K. & Poolman, B. Detergent-mediated reconstitution of membrane proteins. Biochemistry 37, 16410–16415 (1998).
Lévy, D., Gulik, A., Bluzat, A. & Rigaud, J.L. Reconstitution of the sarcoplasmic reticulum Ca(2+)-ATPase: mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. Biochim. Biophys. Acta 1107, 283–298 (1992).
Fang, G. et al. Manipulation of activity and orientation of membrane-reconstituted di-tripeptide transport protein DtpT of Lactococcus lactis. Mol. Membr. Biol. 16, 297–304 (1999).
Rigaud, J.L., Paternostre, M.T. & Bluzat, A. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 2. Incorporation of the light-driven proton pump bacteriorhodopsin. Biochemistry 27, 2677–2688 (1988).
Eytan, G.D. Use of liposomes for reconstitution of biological functions. Biochim. Biophys. Acta 694, 185–202 (1982).
Higgins, C.F. Multiple molecular mechanisms for multidrug resistance transporters. Nature 446, 749–757 (2007).
Davidson, A.L. & Chen, J. ATP-binding cassette transporters in bacteria. Annu. Rev. Biochem. 73, 241–268 (2004).
Biemans-Oldehinkel, E., Doeven, M.K. & Poolman, B. ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett. 580, 1023–1035 (2006).
Neu, H.C. & Heppel, L.A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J. Biol. Chem. 240, 3685–3692 (1965).
Sutcliffe, I.C. & Russell, R.R. Lipoproteins of gram-positive bacteria. J. Bacteriol. 177, 1123–1128 (1995).
Albers, S.V. et al. Glucose transport in the extremely thermoacidophilic Sulfolobus solfataricus involves a high-affinity membrane-integrated binding protein. J. Bacteriol. 181, 4285–4291 (1999).
van der Heide, T. & Poolman, B. ABC transporters: one, two or four extracytoplasmic substrate-binding sites? EMBO Rep. 3, 938–943 (2002).
Patzlaff, J.S., van der Heide, T. & Poolman, B. The ATP/substrate stoichiometry of the ATP-binding cassette (ABC) transporter OpuA. J. Biol. Chem. 278, 29546–29551 (2003).
Biemans-Oldehinkel, E., Mahmood, N.A. & Poolman, B.A sensor for intracellular ionic strength. Proc. Natl. Acad. Sci. USA 103, 10624–10629 (2006).
Mahmood, N.A., Biemans-Oldehinkel, E., Patzlaff, J.S., Schuurman-Wolters, G.K. & Poolman, B. Ion specificity and ionic strength dependence of the osmoregulatory ABC transporter OpuA. J. Biol. Chem. 281, 29830–29839 (2006).
Borths, E.L., Poolman, B., Hvorup, R.N., Locher, K.P. & Rees, D.C. In vitro functional characterization of BtuCD-F, the Escherichia coli ABC transporter for vitamin B12 uptake. Biochemistry 44, 16301–16309 (2005).
Detmers, F.J. et al. Combinatorial peptide libraries reveal the ligand-binding mechanism of the oligopeptide receptor OppA of Lactococcus lactis. Proc. Natl. Acad. Sci. USA 97, 12487–12492 (2000).
Doeven, M.K., Abele, R., Tampé, R. & Poolman, B. The binding specificity of OppA determines the selectivity of the oligopeptide ATP-binding cassette transporter. J. Biol. Chem. 279, 32301–32307 (2004).
van der Heide, T. & Poolman, B. Osmoregulated ABC-transport system of Lactococcus lactis senses water stress via changes in the physical state of the membrane. Proc. Natl. Acad. Sci. USA 97, 7102–7106 (2000).
Schuurman-Wolters, G.K. & Poolman, B. Substrate specificity and ionic regulation of GlnPQ from Lactococcus lactis. An ATP-binding cassette transporter with four extracytoplasmic substrate-binding domains. J. Biol. Chem. 280, 23785–23790 (2005).
Margolles, A., Putman, M., van Veen, H.W. & Konings, W.N The purified and functionally reconstituted multidrug transporter LmrA of Lactococcus lactis mediates the transbilayer movement of specific fluorescent phospholipids. Biochemistry 38, 16298–16306 (1999).
Orelle, C., Dalmas, O., Gros, P., Di Pietro, A. & Jault, J. The conserved glutamate residue adjacent to the Walker-B motif is the catalytic base for ATP hydrolysis in the ATP-binding cassette transporter BmrA. J. Biol. Chem. 278, 47002–47008 (2003).
Jung, H., Tebbe, S., Schmid, R. & Jung, K. Unidirectional reconstitution and characterization of purified Na+/proline transporter of Escherichia coli. Biochemistry 37, 11083–11088 (1998).
Horn, C., Bremer, E. & Schmitt, L. Functional overexpression and in vitro re-association of OpuA, an osmotically regulated ABC-transport complex from Bacillus subtilis. FEBS Lett. 579, 5765–5768 (2005).
Urbani, A. & Warne, T. A colorimetric determination for glycosidic and bile salt-based detergents: applications in membrane protein research. Anal. Biochem. 336, 117–124 (2005).
Strop, P. & Brunger, A.T. Refractive index-based determination of detergent concentration and its application to the study of membrane proteins. Protein. Sci. 14, 2207–2211 (2005).
daCosta, C.J. & Baenziger, J.E. A rapid method for assessing lipid:protein and detergent:protein ratios in membrane-protein crystallization. Acta Crystallogr. D Biol. Crystallogr. 59, 77–83 (2003).
Møller, J.V. & le Maire, M. Detergent binding as a measure of hydrophobic surface area of integral membrane proteins. J. Biol. Chem. 268, 18659–18672 (1993).
Pebay-Peyroula, E. et al. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426, 39–44 (2003).
Biemans-Oldehinkel, E. & Poolman, B. On the role of the two extracytoplasmic substrate-binding domains in the ABC transporter OpuA. EMBO J. 22, 5983–5993 (2003).
van der Heide, T., Stuart, M.C. & Poolman, B. On the osmotic signal and osmosensing mechanism of an ABC transport system for glycine betaine. EMBO J. 20, 7022–7032 (2001).
Newman, M.J. & Wilson, T.H. Solubilization and reconstitution of the lactose transport system from Escherichia coli. J. Biol. Chem. 255, 10583–10586 (1980).
Rigaud, J., Levy, D., Mosser, G. & Lambert, O. Detergent removal by non-polar polystyrene beads. Applications to membrane protein reconstitution and two-dimensional crystallization. Eur. Biophys. J. 27, 305–319 (1998).
Borths, E., Locher, K., Lee, A. & Rees, D. The structure of Escherichia coli BtuF and binding to its cognate ATP binding cassette transporter. Proc. Natl. Acad. Sci. USA 99, 16642–16647 (2002).
Boch, J., Kempf, B. & Bremer, E. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J. Bacteriol. 176, 5364–5371 (1994).
Pidgeon, C., Apostol, G. & Markovich, R. Fourier transform infrared assay of liposomal lipids. Anal. Biochem. 181, 28–32 (1989).
Silhavy, T.J., Szmelcman, S., Boos, W. & Schwartz, M. On the significance of the retention of ligand by protein. Proc. Natl. Acad. Sci. USA 72, 2120–2124 (1975).
Miller, D.M. 3rd, Olson, J.S., Pflugrath, J.W. & Quiocho, F.A. Rates of ligand binding to periplasmic proteins involved in bacterial transport and chemotaxis. J. Biol. Chem. 258, 13665–13672 (1983).
Kragh-Hansen, U., le Maire, M. & Møller, J.V. The mechanism of detergent solubilization of liposomes and protein-containing membranes. Biophys. J. 75, 2932–2946 (1998).
Doeven, M.K. et al. Distribution, lateral mobility and function of membrane proteins incorporated into giant unilamellar vesicles. Biophys. J. 88, 1134–1142 (2005).
Angelova, M.I., Soléau, S., Méléard, P., Faucon, J.F. & Bothorel, P. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. Prog. Colloid Polym. Sci. 89, 127–131 (1992).
Mayer, L.D., Hope, M.J. & Cullis, P.R. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta 858, 161–168 (1986).
MacDonald, R.C. et al. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta 1061, 297–303 (1991).
White, G.F., Racher, K.I., Lipski, A., Hallett, F.R. & Wood, J.M. Physical properties of liposomes and proteoliposomes prepared from Escherichia coli polar lipids. Biochim. Biophys. Acta 1468, 175–186 (2000).
Oku, N., Kendall, D.A. & MacDonald, R.C. A simple procedure for the determination of the trapped volume of liposomes. Biochim. Biophys. Acta 691, 332–340 (1982).
Acknowledgements
The authors greatly acknowledge the past and present members of the Poolman laboratory: without their contributions the protocols presented here would not have been established. The EU-FP6 program (E-MeP; 504601), Netherlands Proteomics Centre (NPC) and the National Science Foundation (NWO-Top Subsidy, grant 700.56.302) are acknowledged for funding.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure 1
Glycine betaine transport by OpuA. [14C]-glycine betaine uptake by OpuA proteoliposomes containing an ARS in 100 mM KPi, pH7.0 without (○; isotonic) or with 250 mM KCl (□; hyperosmotic). The data is corrected for background binding of [14C]-glycine betaine to OpuA liposomes without the ARS. (PDF 805 kb)
Supplementary Figure 2
Demonstration of glycine betaine uptake by RSO-reconstituted OpuA and efflux by ISO-reconstituted OpuA. Following [14C]glycine betaine preloading of OpuA proteoliposomes (containing 10 mM Mg-ATP in the vesicle lumen) under activating conditions (80 mM KPi, pH 7.0 with 450 mM sucrose) and assaying for actual uptake (•), the proteoliposomes were stored on ice for subsequent measurements of glycine betaine efflux via inside-out reconstituted OpuA. Following storage on ice (no leakage of [14C]glycine betaine was observed up to 4 h of storage), samples were diluted 5-fold with 10 mM KPi, pH 7.0, and equilibrated for 3 min at 30 °C. Subsequently, 0.3 M KCl was added to activate OpuA; the addition was done by a 2-fold dilution with pre-warmed medium of the appropriate composition (for further details, see a); this step, the ionic activation, is required for osmoregulatory ABC transporters a, b but will not be necessary for other ABC transport systems. After another 2 min of incubation, 10 mM Mg-ATP was added to energize inside-out oriented OpuA (▪). Samples were taken at the indicated time points and processed in the same way as for uptake measurements. (PDF 264 kb)
Rights and permissions
About this article
Cite this article
Geertsma, E., Nik Mahmood, N., Schuurman-Wolters, G. et al. Membrane reconstitution of ABC transporters and assays of translocator function. Nat Protoc 3, 256–266 (2008). https://doi.org/10.1038/nprot.2007.519
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.519
This article is cited by
-
Structural basis for the activation of the lipid scramblase TMEM16F
Nature Communications (2022)
-
Determining small-molecule permeation through lipid membranes
Nature Protocols (2022)
-
Elucidating the Mechanism Behind Sodium-Coupled Neurotransmitter Transporters by Reconstitution
Neurochemical Research (2022)
-
Stabilization of supramolecular membrane protein–lipid bilayer assemblies through immobilization in a crystalline exoskeleton
Nature Communications (2021)
-
Cryo-EM reveals unique structural features of the FhuCDB Escherichia coli ferrichrome importer
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.