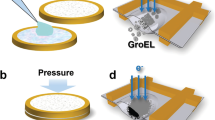
Abstract
This protocol describes the preparation of frozen-hydrated single-particle specimens of macromolecular complexes. First, it describes how to create a grid surface coated with holey carbon by first inducing holes in a Formvar film to act as a template for the holey carbon that is stable under cryo-electron microscopy (cryo-EM) conditions and is sample-friendly. The protocol then describes the steps required to deposit the homogeneous sample on the grid and to plunge-freeze the grid into liquid ethane at the temperature of liquid nitrogen, so that it is suitable for cryo-EM visualization. It takes 4–5 h to make several hundred holey carbon grids and about 1 h to make the frozen-hydrated grids. The time required for sample purification varies from hours to days, depending on the sample and the specific procedure required. A companion protocol details how to collect cryo-EM data using an FEI Tecnai transmission electron microscope that can subsequently be processed to obtain a three-dimensional reconstruction of the macromolecular complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sjoberg, A., Onnerfjord, P., Morgelin, M., Heinegard, D. & Blom, A.M. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J. Biol. Chem. 280, 32301–32308 (2005).
Spahn, C.M. et al. Hepatitis C virus IRES RNA-induced changes in the conformation of the 40s ribosomal subunit. Science 291, 1959–1962 (2001).
Henderson, R. The potential and limitations of neutrons, electrons and X-rays for atomic resolution microscopy of unstained biological molecules. Q. Rev. Biophys. 28, 171–193 (1995).
Halic, M., Becker, T., Frank, J., Spahn, C.M. & Beckmann, R. Localization and dynamic behavior of ribosomal protein L30e. Nat. Struct. Mol. Biol. 12, 467–468 (2005).
Jiang, W. & Ludtke, S.J. Electron cryomicroscopy of single particles at subnanometer resolution. Curr. Opin. Struct. Biol. 15, 571–577 (2005).
Taylor, D.J. et al. Structures of modified eEF2 80S ribosome complexes reveal the role of GTP hydrolysis in translocation. EMBO J 26, 2421–2431 (2007).
Valle, M. et al. Incorporation of aminoacyl-tRNA into the ribosome as seen by cryo-electron microscopy. Nat. Struct. Biol. 10, 899–906 (2003).
Rossmann, M.G. Fitting atomic models into electron-microscopy maps. Acta Crystallogr. D Biol. Crystallogr. 56, 1341–1349 (2000).
Grassucci, R.A., Taylor, D. & Frank, J. Visualization of macromolecular complexes using cryo-electron microscopy with FEI Tecnai TEMs. Nat. Protoc. (in the press) doi: 10.1038/nprot.2007.474.
Gao, H. et al. RF3 induces ribosomal conformational changes responsible for dissociation of class I release factors. Cell 129, 929–941 (2007).
Roseman, A.M., Ranson, N.A., Gowen, B., Fuller, S.D. & Saibil, H.R. Structures of unliganded and ATP-bound states of the Escherichia coli chaperonin GroEL by cryoelectron microscopy. J. Struct. Biol. 135, 115–125 (2001).
Baumeister, W. & Seredynski, J. Preparation of perforated films with predeterminable hole size distributions. Micron 7, 49–54 (1976).
Dubochet, J., Groom, M. & Mueller-Neuteboom, S. The mounting of macromolecules for electron microscopy with particular reference to surface phenomena and treatment of support films by glow discharge. In Advances in Optical and Electron Microscopy (eds. Barrer, R. & Cosslett, V.E.) 107–135 (Academic Press, London, New York, 1982).
Stoffler, G. & Stoffler-Meilicke, M. Immunoelectron microscopy of ribosomes. Annu. Rev. Biophys. Bioeng. 13, 303–330 (1984).
Stöffler, G. & Stöffler-Meilicke, M. The ultrastructure of macromolecular complexes studied with antibodies. In Modern Methods in Protein Chemistry (ed. Tesche, H.) 409–455 (De Gruyter, Berlin, 1983).
Ohi, M., Li, Y., Cheng, Y. & Walz, T. Negative staining and image classification—powerful tools in modern electron microscopy. Biol. Proced. Online 6, 23–34 (2004).
Adrian, M., Dubochet, J., Fuller, S.D. & Harris, J.R. Cryo-negative staining. Micron 29, 145–160 (1998).
Dubochet, J., Lepault, J., Freeman, R., Berriman, J.A. & Homo, J.-C. Electron microscopy of frozen water and aqueous solutions. J. Microsc. 128, 219–237 (1982).
Valle, M. et al. Cryo-EM reveals an active role for aminoacyl-tRNA in the accommodation process. EMBO J 21, 3557–3567 (2002).
Scheres, S.H. et al. Disentangling conformational states of macromolecules in 3D-EM through likelihood optimization. Nat. Methods 4, 27–29 (2007).
Gao, H. et al. Study of the structural dynamics of the E coli 70S ribosome using real-space refinement. Cell 113, 789–801 (2003).
Cyrklaff, M., Adrian, M. & Dubochet, J. Evaporation during preparation of unsupported thin vitrified aqueous layers for cryo-electron microscopy. J. Electron Microsc. Tech. 16, 351–355 (1990).
Iancu, C.V. et al. Electron cryotomography sample preparation using the Vitrobot. Nat. Protoc. 1, 2813–2819 (2007).
White, H.D., Thirumurugan, K., Walker, M.L. & Trinick, J. A second generation apparatus for time-resolved electron cryo-microscopy using stepper motors and electrospray. J. Struct. Biol. 144, 246–252 (2003).
Acknowledgements
We acknowledge support from HHMI, NIH R37 GM29169, R01 GM55440 and P41 RR01219. We also thank M. Watters for assistance in the preparation of the illustrations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grassucci, R., Taylor, D. & Frank, J. Preparation of macromolecular complexes for cryo-electron microscopy. Nat Protoc 2, 3239–3246 (2007). https://doi.org/10.1038/nprot.2007.452
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.452
This article is cited by
-
A large expert-curated cryo-EM image dataset for machine learning protein particle picking
Scientific Data (2023)
-
Ice thickness monitoring for cryo-EM grids by interferometry imaging
Scientific Reports (2022)
-
Sample preparation strategies for efficient correlation of 3D SIM and soft X-ray tomography data at cryogenic temperatures
Nature Protocols (2021)
-
Conformational distortion in a fibril-forming oligomer arrests alpha-Synuclein fibrillation and minimizes its toxic effects
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.