Abstract
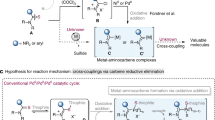
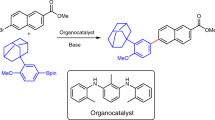
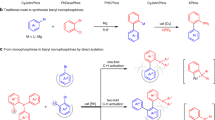
The following protocol describes the application of a highly active Pd-based catalyst system in the Suzuki–Miyaura cross-coupling reaction of arylboronic acids with aryl chlorides to provide biaryl compounds. The general procedure includes a detailed description of an appropriate reaction setup, two methods for assaying the crude reaction mixture (GC and TLC) and a procedure for the isolation, purification and characterization of the anticipated product. Reagents and catalyst precursors can be manipulated in the air; however, the cross-coupling reactions must be performed in an inert atmosphere. Two Suzuki–Miyaura reactions are included in the text as representative examples of these procedures. Although the reactions can proceed in less than 5 min, the protocols, including workup, generally take 6–30 h to be completed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Smith, G.B., Denzeny, G.C., Hughes, D.L., King, A.O. & Verhoeven, T.R. Mechanistic studies of the Suzuki cross-coupling reaction. J. Org. Chem. 59, 8151–8156 (1994).
Jacks, T.E. et al. Development of a scalable process for CI-1034, an endothelin antagonist. Org. Proc. Res. Dev. 8, 201–212 (2004).
Miller, W.D., Fray, A.H., Quatroche, J.T. & Sturgill, C.D. Supression of a palladium-mediated homocoupling in a Suzuki cross-coupling reaction. Development of an impurity control strategy supporting synthesis of LY451395. Org. Proc. Res. Dev. 11, 359–364 (2007).
Ise, T. et al. Light emitting element and azole compound. European patent 1,175,128 A2 (2001).
de Meijere A., Diederich F., (eds.) Metal-Catalyzed Cross-Coupling Reactions 2nd edn. (Wiley-VCH, New York, 2004).
Bellina, F., Carpita, A. & Rossi, R. Palladium catalysts for the Suzuki cross-coupling reaction: an overview of recent advances. Synthesis 2419–2440 (2004).
Littke, A.F & Fu, G. Palladium-catalyzed coupling reactions of aryl chlorides. Angew. Chem. Int. Ed. Engl. 41, 4176–4211 (2002).
Old, D.W., Wolfe, J.P. & Buchwald, S.L. A highly active catalyst for palladium-catalyzed cross-coupling reactions: room-temperature Suzuki couplings and amination of unactivated aryl chlorides. J. Am. Chem. Soc. 120, 9722–9723 (1998).
Walker, S.D., Barder, T.E., Martinelli, J.R. & Buchwald, S.L. A rationally designed universal catalyst for Suzuki–Miyaura coupling processes. Angew. Chem. Int. Ed. Engl. 43, 1871–1876 (2004).
Barder, T.E., Walker, S.D., Martinelli, J.R. & Buchwald, S.L. Catalysts for Suzuki–Miyaura coupling processes: scope and studies of the effect of ligand structure. J. Am. Chem. Soc. 127, 4685–4696 (2005).
Billingsley, K.L., Anderson, K.W. & Buchwald, S.L. A highly active catalyst for Suzuki-Miyaura cross-coupling reactions of heteroaryl compounds. Angew. Chem. Int. Ed. Engl. 45, 3484–3488 (2006).
Billingsley, K.L. & Buchwald, S.L. Highly efficient monophosphine-based catalyst for the palladium-catalyzed Suzuki–Miyaura reaction of heteroaryl halides and heteroaryl boronic acids and esters. J. Am. Chem. Soc. 129, 3358–3366 (2007).
Nguyen, H.N., Huang, X. & Buchwald, S.L. The first general palladium catalyst for the Suzuki–Miyaura and carbonyl enolate coupling of aryl arenesulfonates. J. Am. Chem. Soc. 125, 11818–11819 (2003).
Milne, J.E. & Buchwald, S.L. An extremely active catalyst for the Negishi cross-coupling reaction. J. Am. Chem. Soc. 126, 13028–13032 (2004).
Martin, R. & Buchwald, S.L. Pd-catalyzed Kumada–Corriu cross-coupling reactions at low temperatures allow the use of Knochel-type Grignard reagents. J. Am. Chem. Soc. 129, 3844–3845 (2007).
Anderson, K.W. & Buchwald, S.L. General catalysts for the Suzuki–Miyaura and Sonogashira coupling reactions of aryl chlorides and for the coupling of challenging substrate combinations in water. Angew. Chem. Int. Ed. Engl. 44, 6173–6177 (2005).
Huang, X., Anderson, K.W., Zim, D., Jiang, L., Klapers, A. & Buchwald, S.L. Expanding Pd-catalyzed C-N bond-forming processes: the first amidation of aryl sulfonates, aqueous amination, and complementarity with Cu-catalyzed reactions. J. Am. Chem. Soc. 125, 6653–6655 (2003).
Still, W.C., Kahn, M. & Mitra, A. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 43, 2923–2925 (1978).
Acknowledgements
Funds for this work were provided by the National Institutes of Health (GM 46059). We also acknowledge Merck, Amgen and Boehringer Ingelheim for additional unrestricted fiscal support. We thank BASF for a gift of Pd(OAc)2. R.A.A. thanks Pfizer for a graduate fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altman, R., Buchwald, S. Pd-catalyzed Suzuki–Miyaura reactions of aryl halides using bulky biarylmonophosphine ligands. Nat Protoc 2, 3115–3121 (2007). https://doi.org/10.1038/nprot.2007.411
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.411
This article is cited by
-
A Simple Building Block with Noncovalently Conformational Locks towards Constructing Low-Cost and High-Performance Nonfused Ring Electron Acceptors
Chinese Journal of Polymer Science (2023)
-
Triphenylphosphine-Containing Thermo-Responsive Copolymers: Synthesis, Characterization and Catalysis Application
Macromolecular Research (2019)
-
Synthesis, characterization, and crystal structures of aryl-substituted ferrocenylpyrimidines by site-selective stepwise couplings of 2,4,6-trichloropyrimidine
Monatshefte für Chemie - Chemical Monthly (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.