Abstract
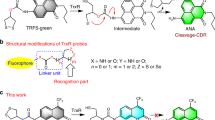
Protein prenylation is one of the most common post-translational modifications affecting hundreds of eukaryotic proteins. Rab geranylgeranyl transferase prenylates exclusively the GTPases of Rab family, and inhibition of this enzyme induces apoptosis in cancer cells, making it an attractive anticancer target. To efficiently test for possible inhibitors of this enzyme, a robust high-throughput assay is required. Here, we present protocols for the synthesis of a fluorescent analogue of geranylgeranyl pyrophosphate NBD-FPP. We utilized this fluorescent probe to design a high-throughput fluorometric assay of Rab prenylation. This continuous fluorometric assay offers the advantage of being sensitive, cost-effective and amendable to miniaturization. The protocol includes the synthesis of the fluorescent substrate, setup of the assay, assay procedure and data analysis. The procedure for the Rab geranylgeranyl transferase (RabGGTase) plate assay depends on the number of compounds in the screen but generally can be performed within a day.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Maurer-Stroh, S., Washietl, S. & Eisenhaber, F. Protein prenyltransferases. Genome Biol. 4 (suppl. 4): 212 (2003).
Casey, P.J. & Seabra, M.C. Protein prenyltransferases. J. Biol. Chem. 271, 5289–5292 (1996).
Farnsworth, C.C., Seabra, M.C., Ericsson, L.H., Gelb, M.H. & Glomset, J.A. Rab geranylgeranyl transferase catalyzes the geranylgeranylation of adjacent cysteines in the small GTPases Rab1A, Rab3A, and Rab5A. Proc. Natl. Acad. Sci. USA 91, 11963–11967 (1994).
Alexandrov, K., Horiuchi, H., Steele-Mortimer, O., Seabra, M.C. & Zerial, M. Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated rab proteins to their target membranes. EMBO J. 13, 5262–5273 (1994).
Andres, D.A. et al. cDNA cloning of component A of Rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell 73, 1091–1099 (1993).
Seabra, M.C., Brown, M.S., Slaughter, C.A., Sudhof, T.C. & Goldstein, J.L. Purification of component A of Rab geranylgeranyl transferase: possible identity with the choroideremia gene product. Cell 70, 1049–1057 (1992).
Gibbs, J.B. Ras C-terminal processing enzymes—new drug targets? Cell 65, 1–4 (1991).
James, G.L. et al. Benzodiazepine peptidomimetics: potent inhibitors of Ras farnesylation in animal cells. Science 260, 1937–1942 (1993).
Singh, S.B. & Lingham, R.B. Current progress on farnesyl protein transferase inhibitors. Curr. Opin. Drug Discov. Dev. 5, 225–244 (2002).
Gelb, M.H. et al. Therapeutic intervention based on protein prenylation and associated modifications. Nat. Chem. Biol. 2, 518–528 (2006).
Head, J.E. & Johnston, S.R. Protein farnesyltransferase inhibitors. Expert. Opin. Emerg. Drugs 8, 163–178 (2003).
Prendergast, G.C. Actin' up: RhoB in cancer and apoptosis. Nat. Rev. Cancer 1, 162–168 (2001).
Lackner, M.R. et al. Chemical genetics identifies Rab geranylgeranyl transferase as an apoptotic target of farnesyl transferase inhibitors. Cancer Cell 7 (suppl. 4): 325–336 (2005).
Roelofs, A.J. et al. Selective inhibition of Rab prenylation by a phosphonocarboxylate analogue of risedronate induces apoptosis, but not S-phase arrest, in human myeloma cells. Int. J. Cancer 119, 1254–1261 (2006).
Coxon, F.P. et al. Identification of a novel phosphonocarboxylate inhibitor of Rab geranylgeranyl transferase that specifically prevents Rab prenylation in osteoclasts and macrophages. J. Biol. Chem. 276, 48213–48222 (2001).
Pompliano, D.L., Gomez, R.P. & Anthony, N.J. Intramolecular fluorescence enhancement—a continuous assay of ras farnesyl–protein transferase. J. Am. Chem. Soc. 114, 7945–7946 (1992).
Pickett, W.C. et al. A fluorescence assay for geranylgeranyl transferase type I. Anal. Biochem. 225, 60–63 (1995).
Anant, J.S. et al. Mechanism of Rab geranylgeranylation: formation of the catalytic ternary complex. Biochemistry 37, 12559–12568 (1998).
Thoma, N.H., Niculae, A., Goody, R.S. & Alexandrov, K. Double prenylation by RabGGTase can proceed without dissociation of the mono-prenylated intermediate. J. Biol. Chem. 276, 48631–48636 (2001).
Durek, T. et al. Synthesis of fluorescently labeled mono- and diprenylated Rab7 GTPase. J. Am. Chem. Soc. 126, 16368–16378 (2004).
Troutman, J.M., Roberts, M.J., Andres, D.A. & Spielmann, H.P. Tools to analyze protein farnesylation in cells. Bioconjug. Chem. 16, 1209–1217 (2005).
Turek, T.C., Gaon, I., Gamache, D. & Distefano, M.D. Synthesis and evaluation of benzophenone-based photoaffinity labeling analogs of prenyl pyrophosphates containing stable amide linkages. Bioorg. Med. Chem. Lett. 7, 2125–2130 (1997).
Dursina, B. et al. Identification and specificity profiling of protein prenyltransferase inhibitors using new fluorescent phosphoisoprenoids. J. Am. Chem. Soc. 128, 2822–2835 (2006).
Wu, Y.W. et al. A protein fluorescence amplifier: continuous fluorometric assay for rab geranylgeranyltransferase. ChemBioChem 7, 1859–1861 (2006).
Rose, M.W. et al. Evaluation of geranylazide and farnesylazide diphosphate for incorporation of prenylazides into a CAAX box-containing peptide using protein farnesyltransferase. J. Pept. Res. 65, 529–537 (2005).
Davisson, V.J. et al. Phosphorylation of isoprenoid alcohols. J. Org. Chem. 51, 4768–4779 (1986).
Kalinin, A. et al. Expression of mammalian geranylgeranyltransferase type-II in Escherichia coli and its application for in vitro prenylation of Rab proteins. Protein Expr. Purif. 22, 84–91 (2001).
Acknowledgements
K.A. was supported by a Heisenberg Award of the Deutsche Forschungsgemeinschaft. L.B. was supported by a Sofja Kovalevskaja Award of the Alexander von Humboldt Foundation. This work was supported in part by grant DFG AL 484/7-2 to K.A. and grant SFB642 of the Deutsche Forschungsgemeinschaft to K.A., Roger S. Goody and Herbert Waldmann.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Data
Analytical data for the synthesis of a fluorescent analogue of geranylgeranyl pyprophosphate (DOC 54 kb)
Rights and permissions
About this article
Cite this article
Wu, YW., Alexandrov, K. & Brunsveld, L. Synthesis of a fluorescent analogue of geranylgeranyl pyrophosphate and its use in a high-throughput fluorometric assay for Rab geranylgeranyltransferase. Nat Protoc 2, 2704–2711 (2007). https://doi.org/10.1038/nprot.2007.401
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.401
This article is cited by
-
Proof of concept for AAV2/5-mediated gene therapy in iPSC-derived retinal pigment epithelium of a choroideremia patient
Molecular Therapy - Methods & Clinical Development (2014)
-
The analytical determination of isoprenoid intermediates from the mevalonate pathway
Analytical and Bioanalytical Chemistry (2012)
-
Structures of RabGGTase–substrate/product complexes provide insights into the evolution of protein prenylation
The EMBO Journal (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.