Abstract
This protocol describes detailed procedures for the preparation of a rhodamine-based mercury probe and for its applications to the detection of mercury in cells and vertebrate organisms. The mercury probe 1, which is prepared in two steps from rhodamine 6G, responds rapidly to Hg2+ in aqueous solutions with a 1:1 stoichiometry. Owing to the fact that the probe reacts with Hg2+ in an irreversible manner, it has advantages over other reversible mercury probes in in vivo assays with respect to both sensitivity and selectivity. In addition, fluorescent imaging assays of Hg2+ in live cells and zebrafish by using this mercury probe are detailed in this protocol. The approximate time frame for the preparation of the probe is 24 h and for its use in imaging assays is 1.5 h.
Similar content being viewed by others
Introduction
Mercury, a highly toxic metal, can easily pass through biological membranes1. Mercury absorbed in human bodies causes serious damage to tissues and organs2. Long-term exposure to high levels of mercury results in deterioration of the brain and kidneys, while short-term exposure to high levels of this metal leads to lung damage, nausea, vomiting and diarrhea. Despite the serious toxic effects on biological systems, efficient and sensitive in vivo monitoring methods for mercury have not been fully developed3. Because of their high sensitivity, simplicity and reproducibility, fluorescent chemosensor techniques4,5,6,7,8 have been extensively employed for the detection of biologically important metal ions. However, most of the known mercury fluorescent chemosensors9,10,11 do not meet the selectivity and sensitivity requirements for the in vivo, aqueous solution imaging assays.
The recently uncovered, rhodamine-based fluorescent probe 1 (Fig. 1) has been found to be ideally suited for the in vivo monitoring of mercury ions because of its high sensitivity and selectivity. This protocol describes the method for preparation of the fluorescent probe 1 (Fig. 1)12, and its application to monitoring of Hg2+ in living cells and zebrafish as a model of vertebrate organisms13. Unlike other probes, which operate by reversible binding to metal ions, the response of this probe to Hg2+ is associated with an irreversible chemical reaction14. In general, the signal intensities of reversible probes depend on the position of the binding equilibrium, which is sensitive to the environmental conditions, such as pH and the presence of other chelating molecules. However, those of irreversible probes are proportional to the extent of the chemical conversions not to the equilibrium positions, and therefore are less sensitive to the environmental conditions compared with the reversible cases. As a consequence, irreversible probes are more suitable for in vivo assays than reversible probes with respect to their high selectivity and sensitivity. Probe 1, prepared from rhodamine 6G in high yield by a two-step procedure15, has several advantageous features. These include the fact that non-fluorescent 1 reacts irreversibly with Hg2+ in a 1:1 stoichiometric fashion to produce the 1,3,4-oxadiazole 3 (Fig. 2), a substance that is strongly fluorescent at long wavelengths (emission at 557 nm) where background autofluorescence in cells is minimal. In addition, the probe shows a high selectivity to Hg2+ over other biologically relevant metal ions (see Table 1 for the selectivity profile of 1) and a high sensitivity for Hg2+ in aqueous solutions (1 ppb of Hg2+ in aqueous solutions can be detected by this probe). Moreover, in addition to the above-mentioned features, its high cell permeability makes it possible to employ 1 to detect Hg2+ in living cells and, in particular, vertebrate organisms. Fluorescence images of the cells and zebrafish that are treated with Hg2+ and then incubated with the mercury probe 1 allow rapid in vivo assays of Hg2+. In vivo imaging of Hg2+ by using fluorescent probe technique can provide important information about the site-specific accumulation and real-time monitoring of Hg2+ uptake.
Materials
Reagents
-
Rhodamine 6G (Sigma-Aldrich, cat. no. R4127)
-
Hydrazine monohydrate (Sigma-Aldrich, cat. no. 207942)
Caution
Toxic and dangerous for the environment (see material safety data sheet at http://www.sigmaaldrich.com/catalog/search/ ProductDetail/SIAL/207942).
-
Phenyl isothiocyanate (Sigma-Aldrich, cat. no. 139742)
Caution
Highly toxic, combustible, flammable and harmful if swallowed (see material safety data sheet at http://www.sigmaaldrich.com/catalog/search/ ProductDetail/ALDRICH/139742).
-
Mercury(II) chloride (Sigma-Aldrich, cat. no. 203777)
Caution
Highly toxic and dangerous for the environment (see material safety data sheet at http://www.sigmaaldrich.com/catalog/search/ ProductDetail/ALDRICH/203777).
-
Sodium hydroxide (Samchun Chemicals, cat. no. 122401)
-
HPLC grade methanol (Burdick & Jackson, cat. no. AH230)
-
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, cat. no. 276855)
-
Anhydrous N,N-dimethylformamide (Sigma-Aldrich, cat. no. 227056)
-
HPLC grade dichloromethane (Burdick & Jackson, cat. no. AH300)
-
HPLC grade ethyl acetate (Burdick & Jackson, cat. no. AS100)
-
HPLC grade hexanes (Burdick & Jackson, cat. no. GC 217)
-
Anhydrous magnesium sulfate (Duksan Chemicals, cat. no. A402131)
-
DMEM (Invitrogen, cat. no. 11995)
-
FBS (Sigma-Aldrich, cat. no. 12103C)
-
Trypsin-EDTA solution (Sigma-Aldrich, cat. no. T4174)
-
100 × penicillin/streptomycin (Invitrogen, cat. no. 15140)
-
Phosphate-buffered saline, 1 × sterile solution (PBS, pH 7.4) (Amresco, cat. no. K812)
Equipment
-
Hotplate magnetic stirrer with contact thermometer (Corning)
-
Vacuum pump (Hitachi)
-
Rotary evaporator (Heidolph)
-
Balance (OHAUS)
-
Hand-held UV lamp (Vilber Lourmat)
-
Rubber septum (Sigma-Aldrich, cat. no. Z553964)
-
Disposable glass Pasteur pipettes (Hilgenberg-Gmbh, 150 and 230 mm)
-
Pipette bulbs (Sigma-Aldrich, cat. no. Z136069)
-
Pyrex one-necked round-bottomed flasks (25 ml)
-
Pyrex two-necked round-bottomed flasks (25 ml)
-
Pyrex separatory funnel
-
Pyrex filter funnel
-
Filter paper (Fisher, cat. no. 09-801C)
-
Pyrex chromatography column (Changyoung)
-
Syringe needle (Hamilton, cat. no. 81320/81000)
-
Glass syringe (Hamilton)
-
Disposable polypropylene syringe (HENKE SASS WOLF GMBH)
-
Mercury-free thermometer (Sigma-Aldrich, cat. no. Z561541)
-
KBr IR cell (Sigma-Aldrich, cat. no. Z112119)
-
Heat gun (Daihan-Scientific, cat. no. BOGHG630)
-
Melting point apparatus (Sigma-Aldrich, cat. no. Z289078)
-
Teflon-coated magnetic stirring bar (Sigma-Aldrich, cat. no. Z126969)
-
NMR tube (Sigma-Aldrich, cat. no. NORS55008)
-
NMR (Bruker DRX-250 spectrometer)
-
Avatar 360 FT-IR (Nicolet)
-
Silica gel 230–400 mesh (Merck, cat. no. TA1363285)
-
Silica gel 60 F254 thin-layer chromatography (TLC) plate (Merck, cat. no. OB549444)
-
Petri dish (90 mm) (SPL, cat. no. 10090)
-
Hemacytometer (Superior, cat. no. HSU-1401)
-
Fluorescence microscope (Nicon Eclipse TE2000)
-
Charged-coupled device camera (Lumenera, Infinity-2)
-
Dissection microscope (Stemi 2000-c, ZAISS)
-
Incubator for mammalian cell culture (Sanyo, MCO-5AC)
-
Clean bench (Samki-Lab, cat. no. CB2012-840)
-
Incubator for zebrafish (Jeio, cat. no. ON-02G)
-
Centrifuge (Eppendorf, 5804R)
-
Centrifuge tube (15 ml) (Corning, cat. no. 430719)
-
Culture flask (25 cm2) (Nunc, cat. no. 136196)
-
Multi-well (six-well) plate (Nunc, cat. no. 140675)
-
96-well microplate (Nunc, cat. no. 167008)
-
Mating box (Fig. 3, fabricated by our group)
Figure 3: Figures of a mating box (mesh size: 0.3 cm). -
Disposable transfer pipette (Simport, cat. no. P200-72)
Reagent setup
-
Culture media DMEM supplemented with 10% FBS, 50 U ml−1 of penicillin and 50 μg ml−1 of streptomycin.
-
E3 media 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4 and 10−5% (vol/vol) methylene blue.
Equipment setup
-
When dried round-bottomed flasks are needed for the procedure (see below), store the relevant glassware in the 120 °C oven for at least 3 h. Before using the dried glassware, allow it to cool to room temperature (25 °C) on the bench top for 5 min.
-
Fluorescence microscope The adduct 3 obtained from reaction of mercury probe 1 and Hg2+ exhibits absorption maximum at 500 nm and emission maximum at 557 nm. To detect mercury ions in cells and zebrafish using mercury probe 1, fluorescence is excited by light from a mercury lamp filtered at wavelength 510–560 nm and emission is collected at wavelength 590 nm by a charged-coupled device camera.
Procedure
Synthesis of 2-(6-ethylamino-3-ethylimino-2,7-dimethyl-3H-xanthen-9-yl)benzoic acid hydrazide (2)
-
1
Connect an oven-dried two-necked round-bottomed flask (25 ml) containing a Teflon-coated magnetic stirring bar with a reflux condenser. Close the openings of the round-bottomed flask and the reflux condenser with rubber septa and fill the flask with argon gas.
-
2
Add 300 mg rhodamine 6G (0.63 mmol, 1.0 equiv.) in 2 ml methanol by using a disposable syringe.
-
3
Add 0.10 ml hydrazine monohydrate (1.9 mmol, 3.0 equiv.) to the flask by using a disposable syringe.
-
4
Stir the solution at reflux for 6 h under an argon atmosphere.
-
5
Cool the reaction mixture to room temperature and add 30 ml ethyl acetate.
-
6
Transfer the solution into a separatory funnel and dilute it with 50 ml water. Separate the organic layer and extract the aqueous layer with 10 ml ethyl acetate three times (total volume of ethyl acetate: 30 ml). Wash the combined organic layers with 10 ml of 1 M sodium hydroxide and 5 ml brine (NaCl-saturated aqueous solution).
-
7
Dry the organic layer over anhydrous magnesium sulfate (400 mg) for 15 min with gentle stirring and filter through a fritted glass funnel under an aspirator vacuum. Wash the solids with 25 ml ethyl acetate twice.
-
8
Concentrate the combined filtrates at 25 °C by using a rotary evaporator under vacuum (2 mm Hg).
Pause point
The crude product can be stored at 4 °C overnight.
-
9
Pack a chromatography column (1.5 cm i.d. × 15 cm length) with silica gel using hexanes.
-
10
Load the crude mixture from Step 8 onto the silica bed (approximately 0.5 cm thick).
-
11
Elute the product with a 10:2:1 (vol/vol/vol) mixture of hexanes, dichloromethane and methanol.
-
12
Identify the fractions containing the product by silica gel TLC, developing with a 10:1 mixture of dichloromethane and methanol (Rf = 0.51 for 2, Rf = 0.90 for rhodamine 6G). The product is visualized by UV absorbance at 254 nm.
-
13
Collect the fractions containing the desired product and evaporate the solvent at 25 °C by using a rotary evaporator under vacuum (2 mm Hg) to give rhodamine hydrazide 2 (243 mg, 0.57 mmol, 90%) as a white solid.
Pause point
The product can be stored at −25 °C for several weeks.
Synthesis of 2-(6-ethylamino-3-ethylimino-2,7-dimethyl-3H-xanthen-9-yl)benzoic acid phenylthiourea (1)
-
14
Fit an oven-dried one-necked round-bottomed flask (25 ml) containing a Teflon-coated magnetic stirring bar and close the flask with a septum and fill the flask with argon gas after having briefly set it under vacuum.
-
15
Add 200 mg rhodamine hydrazide 2 (0.47 mmol, 1.0 equiv.) in 3 ml N,N-dimethylformamide by using a disposable syringe.
-
16
Add 0.10 ml phenyl isothiocyanate (0.65 mmol, 1.4 equiv.) to the flask by using a disposable syringe.
-
17
Stir the reaction mixture for 6 h at room temperature under an argon atmosphere.
-
18
Dilute the reaction mixture with 30 ml ethyl acetate and transfer the solution to a separatory funnel.
-
19
Dilute the solution with 30 ml water and separate the organic layer. Extract the aqueous layer with 20 ml ethyl acetate three times and wash the combined organic layers with brine.
-
20
Dry the organic layer over anhydrous magnesium sulfate (400 mg) for 15 min with gentle stirring and filter through a fritted glass funnel under aspirator vacuum. Wash the solids with 25 ml ethyl acetate twice.
-
21
Concentrate the combined filtrates at 25 °C by using a rotary evaporator under vacuum (2 mm Hg).
Pause point
The crude product can be stored at 4 °C overnight.
-
22
Pack a chromatography column (1.5 cm i.d. × 15 cm length) with silica gel using hexanes.
-
23
Load the crude mixture from Step 21 onto the top of the silica gel bed.
-
24
Elute the products with a 4:1:1 (vol/vol/vol) mixture of hexanes, dichloromethane and ethyl acetate.
-
25
Identify the fractions containing the desired product by silica gel TLC, developing with a 2:1 mixture of hexanes and ethyl acetate (Rf = 0.5 for 3, Rf = 0.10 for 2). The product is visualized by UV absorbance at 254 nm.
-
26
Collect the fractions containing the product and evaporate the solvent at 25 °C using a rotary evaporator under vacuum (2 mm Hg) to give the mercury probe 1 (238 mg, 0.42 mmol, 90%) as a white solid.
Pause point
This material can be stored at −25 °C for several weeks in the dark.
Detection of mercury ions in mammalian cells
-
27
Incubate murine C2C12 cells (2.5 × 106 cells) in a culture flask (25 cm2) containing 8 ml culture media inside an incubator filled with 5% CO2 for 24 h at 37 °C.
Critical Step
Cells should be incubated in an incubator supplemented with 5% CO2 at 37 °C to maintain the pH of medium for growth and survival. In general, 5% CO2 is used with 1.2–2.2 g liter−1 of sodium bicarbonate in culture medium to buffer the pH of the medium.
-
28
Remove culture media from the cell culture and wash the cells with 8 ml culture media.
-
29
Add 8 ml culture media to cell culture and incubate the cells until they are 60–90% confluent (usually 24–48 h).
-
30
Remove the culture media from the cell culture and wash the cells with 8 ml sterilized PBS (pH 7.4).
-
31
Treat the cell culture with 2 ml Trypsin-EDTA solution to detach cells from the surface of the culture flask and incubate the cells until the cell layer is dispersed (usually 5–15 min).
-
32
Add 6–8 ml culture media to the cell culture to stop the trypsin reaction, and transfer the cell culture to a 15 ml centrifuge tube.
-
33
Centrifuge the cells at 180g at 25 °C for 5 min and discard the supernatant.
-
34
Suspend the cell pellet in culture media and count the number of cells by using a hematometer.
-
35
Seed 104 C2C12 cells per well in a six-well plate.
Pause point
Incubate the cells in 2 ml culture media for 24 h at 37 °C. Cells are attached to the plate surface during this time.
-
36
Treat the cells with 2 μl of 10 mM mercury chloride (final concentration: 10 μM) dissolved in sterilized PBS (pH 7.4) and incubate for 10 min at 37 °C.
-
37
Wash the treated cells three times with 2 ml PBS to remove the remaining mercury ions. (The exact extent of incorporation of mercury ions into cells is difficult to measure.)
-
38
Add 2 ml culture media to the cell culture and treat the cell culture with 2 μl of 10 mM mercury probe 1 (final concentration: 10 μM) dissolved in DMSO. Incubate for 30 min at 37 °C.
-
39
Obtain fluorescence images (Fig. 4) by using a Nikon TE2000 fluorescence microscope. There is no need to remove the remaining mercury probe 1 from the media as it is not fluorescent.
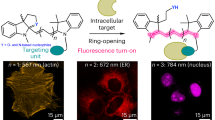
Figure 4: Images of cells and zebrafish incubated with HgCl2 and mercury probe 1. (a) Fluorescence image of C2C12 cells treated with 1 (10 μM) in the absence of external Hg2+ (scale bar, 50 μm). (b) Fluorescence image of C2C12 cells treated with both Hg2+ (10 μM) and 1 (10 μM) (scale bar, 50 μm). (c) Images of a 3-day-old zebrafish treated with 1 (10 μM) in the absence of external Hg2+ (top, microscopic image; bottom, fluorescence microscopic image) (scale bar, 200 μm). (d) Images of a 3-day-old zebrafish treated with both Hg2+ (10 μM) and 1 (10 μM) (top, microscopic image; bottom, fluorescence microscopic image) (scale bar, 200 μm).
Detection of mercury ions in zebrafish
-
40
Transfer a pair of adult zebrafish (3 months old) into a water-filled mating box (Fig. 3) by using a small net and cover the box with a lid to prevent the fish from jumping out. Be careful not to injure the fish during the transfer process. Note that the adult zebrafish is purchased from a local pet store. See ref. 16 for detailed procedures for zebrafish maintenance and breeding.
Critical Step
Zebrafish should be raised at 25–28 °C (optimal temperature: 28 °C) and the aquarium should be clean to keep the fish in healthy breeding conditions.
-
41
Place the mating box in an incubator at 28 °C keeping the inside of the incubator dark.
Pause point
Incubate the mating box under these conditions overnight.
-
42
Remove the mating box from the incubator early in the morning and place the box in the light at 25–28 °C. Frequently check the mating box for eggs.
-
43
Once the fish spawns eggs, remove a top container and collect the embryos from the bottom container.
-
44
Transfer the embryos into 90 mm Petri dish filled with E3 media using a disposable transfer pipette.
Pause point
Raise the embryos in the incubator at 28 °C for 3 days, replenishing the E3 media every day.
Critical Step
Dead embryos should be removed during incubation, as they induce the death of adjacent live embryos.
-
45
Transfer the 3-day-old zebrafish into a 96-well microplate by using a disposable transfer pipette (final volume of E3 media: 100 μl) and add 0.1 μl of 10 mM mercury chloride (final concentration: 10 μM) dissolved in E3 media.
-
46
Incubate the fish for 10 min at 28 °C, remove the media solution and wash the fish three times with 100 μl of E3 media to remove the remaining mercury ions.
-
47
Add 100 μl of E3 media to the mercury-exposed zebrafish and treat the fish with 0.1 μl of 10 mM mercury probe 1 (final concentration: 10 μM) dissolved in DMSO and incubate for 30 min at 37 °C.
-
48
Obtain images (Fig. 4d) by using a Nikon TE2000 fluorescence microscope. There is no need to remove the remaining mercury probe 1 from the media since it is not fluorescent.
Timing
Steps 1–4: 7 h
Steps 5–8: 1 h
Steps 9–13: 4 h
Steps 14–17: 7 h
Steps 18–21: 1 h
Steps 22–26: 4 h
Step 27: 1 day
Steps 28 and 29: 1–3 days
Steps 30–34: 30 min
Step 35: 1 day
Steps 36–39: 1.5 h
Steps 40–44: 3 days and 14 h
Steps 45–48: 1.5 h
Anticipated results
Typical yields
The typical isolated yield of 2 (Steps 1–13) is 90% and the typical isolated yield of mercury probe 1 from 2 (Steps 14–26) is 90%. Typical overall yield of mercury probe 1 from rhodamine 6G (Steps 1–26) is 80%.
Analytical data
2-(6-ethylamino-3-ethylimino-2,7-dimethyl- 3H -xanthen-9-yl)benzoic acid hydrazide ( 2)
White solid, mp 280–282 °C; TLC (dichloromethane/methanol 10:1 vol/vol) Rf = 0.51; 1H NMR (CDCl3, 250 MHz) δ 7.96–7.93 (m, 1H), 7.45–7.42 (m, 2H), 7.07–7.05 (m, 1H), 6.39 (s, 2H), 6.23 (s, 2H), 3.58–3.55 (m, 4H), 3.21 (q, J = 7.3 Hz, 4H), 1.91 (s, 6H), 1.31 (t, J = 7.1 Hz, 6H); 13C NMR (62.9 MHz, CDCl3) δ 166.2, 152.3, 151.8, 147.6, 132.6, 129.9, 128.1, 127.7, 123.8, 123.0, 118.0, 104.9, 96.9, 66.1, 38.4, 16.7, 14.8; IR (film, cm−1) 3,422, 2,969, 2,926, 2,864, 1,692, 1,621, 1,517, 1,468, 1,421, 1,347, 1,270, 1,216, 1,157, 1,011; ESI-MS m/z calculated for C26H29N4O2 [M+H]+ 429.22, found 429.22.
2-(6-ethylamino-3-ethylimino-2,7-dimethyl- 3H -xanthen-9-yl)benzoic acid phenyl thiourea ( 1)
White solid, mp 150–152 °C; TLC (hexane/EtOAc 1:1 vol/vol) Rf = 0.5; 1H NMR (CDCl3, 250 MHz) δ 8.06–8.03 (m, 1H), 7.66–7.57 (m, 3H), 7.26–7.22 (m, 3H), 3.26–3.17 (m, 4H), 1.84 (s, 6H), 1.32 (t, J = 7.1 Hz, 6H); 13C NMR (62.9 MHz, CDCl3) δ 182.2, 166.9, 152.5, 150.8, 148.4, 138.1, 134.1, 129.5, 129.0, 128.2, 128.1, 125.8, 125.2, 124.4, 123.2, 118.9, 104.4, 95.9, 67.0, 37.9, 16.1, 13.7; IR (film, cm−1) 3,326, 2,964, 2,960, 2,950, 2,356, 2,351, 1,713, 1,620, 1,517, 1,430, 1,424, 1,347, 1,274, 1,212, 1,089, 1,016; HRMS (FAB) m/z calculated for C33H33N5O2S [M+H]+ 564.2423, found 564.2433.
Probe 1 shows no fluorescence property and its absorbance maximum is at 538 nm.
Detection of mercury ions in cells and zebrafish
The mercury probe 1 can be used to monitor mercury ions in various cells including mammalian cell lines, differentiated cells and primary cells, and in vertebrate organisms by using fluorescent imaging assays. Data on the in vivo detection of mercury are given in Figure 4. Figure 4b demonstrates that intracellular mercury ions in murine C2C12 myoblasts can be detected by incubating the cells with mercury ion (10 μM) and then with the mercury probe 1 (10 μM). More interesting (Fig. 4d) is the fact that mercury ions in zebrafish can also be readily detected by employing this probe.
Change history
09 August 2007
In the version of this article initially published, the structure of rhodamine 6G in Figure 1 was incorrect. The figure has been replaced in the HTML and PDF versions of the article.
References
Gutknecht, J. Inorganic mercury (Hg2+) transport through lipid bilayer membranes. J. Membr. Biol. 61, 61–66 (1981).
Clarkson, T.W., Magos, L. & Myers, G.J. The toxicology of mercury—current exposures and clinical manifestations. N. Engl. J. Med. 349, 1731–1737 (2003).
Yoon, S., Albers, A.E., Wong, A.P. & Chang, C.J. Screening mercury levels in fish with a selective fluorescent chemosensor. J. Am. Chem. Soc. 127, 16030–16031 (2005).
Valeur, B. Molecular Fluorescence: Principles and Applications (Wiley-VCH, Weinheim, 2002).
Haugland, R.P. (ed.) The Handbook—A Guide to Fluorescent Probes and Labeling Technologies 10th edn. (Molecular Probes, Eugene, OR, 2005).
de Silva, A.P. et al. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97, 1515–1566 (1997).
Fabbrizzi, L. & Poggi, A. Sensors and switches from supramolecular chemistry. Chem. Soc. Rev. 24, 197–202 (1995).
Czarnik, A.W. Chemical communication in water using fluorescent chemosensors. Acc. Chem. Res. 27, 302–308 (1994).
Zhu, X.J., Fu, S.T., Wong, W.K., Guo, J.P. & Wong, W.Y. A near-infrared-fluorescent chemodosimeter for mercuric ion based on an expanded porphyrin. Angew. Chem. Int. Ed. 45, 3150–3154 (2006).
Zheng, H., Qian, Z.H., Xu, L., Yuan, F.F., Lan, L.D. & Xu, J.G. Switching the recognition preference of rhodamine B spirolactam by replacing one atom: design of rhodamine B thiohydrazide for recognition of Hg(II) in aqueous solution. Org. Lett. 8, 859–861 (2006).
Song, K.C. et al. Fluorogenic Hg2+-selective chemodosimeter derived from 8-hydroxyquinoline. Org. Lett. 8, 3413–3416 (2006).
Yang, Y.K., Yook, K.J. & Tae, J. A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J. Am. Chem. Soc. 127, 16760–16761 (2005).
Ko, S.K., Yang, Y.K., Tae, J. & Shin, I. In vivo monitoring of mercury ions using a rhodamine-based molecular probe. J. Am. Chem. Soc. 128, 14150–14155 (2006).
Chae, M.Y. & Czarnik, A.W. Fluorimetric chemodosimetry. Mercury(II) and silver(II) indication in water via enhanced fluorescence signaling. J. Am. Chem. Soc. 114, 9704–9705 (1992).
Yang, X.F., Guo, X.Q. & Zhao, Y.B. Development of a novel rhodamine-type fluorescent probe to determine peroxynitrite. Talanta 57, 883–890 (2002).
Nusslein-Volhard, C. & Dahm, R. Zebrafish. A Practical Approach (Oxford University Press, Oxford, UK, 2002).
Acknowledgements
This work was supported by the SRC programs (R11-2000-070 and R11-2003-019) and the NRL program of MOST/KOSEF. Y.-K.Y. and S.-K.K. thank the BK21 program (KRF).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yang, YK., Ko, SK., Shin, I. et al. Synthesis of a highly metal-selective rhodamine-based probe and its use for the in vivo monitoring of mercury. Nat Protoc 2, 1740–1745 (2007). https://doi.org/10.1038/nprot.2007.246
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.246
This article is cited by
-
Cadmium uptake and oxidative-stress-induced DNA alterations in the freshwater cladoceran Moina macrocopa (Straus 1820) following consecutive short-term exposure assessments
Limnology (2023)
-
Oligonucleotide-based biosensors for in vitro diagnostics and environmental hazard detection
Analytical and Bioanalytical Chemistry (2016)
-
Synthesis of a highly Zn2+-selective cyanine-based probe and its use for tracing endogenous zinc ions in cells and organisms
Nature Protocols (2014)
-
Dual-rhodamine urea derivative, a novel chemidosimeter for Hg(II) and its application in imaging Hg(II) in living cells
JBIC Journal of Biological Inorganic Chemistry (2009)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.