Abstract
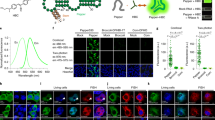
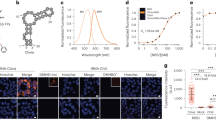
This protocol outlines a methodology for the preparation and characterization of three RNA-specific fluorescent probes (E36, E144 and F22) and their use in live cell imaging. It describes a detailed procedure for their chemical synthesis and purification; serial product characterization and quality control tests, including measurements of their fluorescence properties in solution, measurement of RNA specificity and analysis of cellular toxicity; and live cell staining and counterstaining with Hoechst or DAPI. Preparation and application of these RNA imaging probes takes 1 week.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Johnson, I. Fluorescent probes for living cells. Histochem. J. 30, 123–140 (1998).
Martin, R.M., Leonhardt, H. & Cardoso, M.C. DNA labeling in living cells. Cytometry Part A 67A, 45–52 (2005).
Krishan, A. & Dandekar, P.D. DAPI fluorescence in nuclei isolated from tumors. J. Histochem. Cytochem. 53, 1033–1036 (2005).
Pendergrass, W., Wolf, N. & Poot, M. Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry Part A 61, 162–169 (2004).
Rustom, A., Saffrich, R., Markovic, I., Walther, P. & Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010 (2004).
Mironov, S.L., Ivannikov, M.V. & Johansson, M. [Ca2+]i signaling between mitochondria and endoplasmic reticulum in neurons is regulated by microtubules From mitochondrial permeability transition pore to Ca2+-induced Ca2+ release. J. Biol. Chem. 280, 715–721 (2005).
Mhlanga, M.M., Vargas, D.Y., Fung, C.W., Kramer, F.R. & Tyagi, S. tRNA-linked molecular beacons for imaging mRNAs in the cytoplasm of living cells. Nucleic Acid Res. 33, 1902–1912 (2005).
Femino, A.M., Fay, F.S., Fogarty, K. & Singer, R.H. Visualization of single RNA transcripts in situ. Science 280, 585–590 (1998).
Dirks, R.W., Molenaar, C. & Tanke, H.J. Visualizing RNA molecules inside the nucleus of living cells. Methods 29, 51–57 (2003).
Andersen, J.S. et al. Nucleolar proteome dynamics. Nature 433, 77–83 (2005).
Bertrand, E. et al. Localization of ASH1 mRNA particles in living yeast. Mol. Cell 2, 437–445 (1998).
Li, Q. et al. RNA-selective, live cell imaging probes for studying nuclear structure and function. Chem. Biol. 13, 615–623 (2006).
Lzkowicz, J.R. Principles of Fluorescence Spectroscopy 2nd edn. (Kluwer Academic/Plenum Publishers, New York, USA, 1999).
Fery-Forgues, S. & Lavabre, D. Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products. J. Chem. Educ. 76, 1260–1264 (1999).
Acknowledgements
We gratefully acknowledge the support of the National Science Foundation (CHE-0449139) and for equipment grants for the NMR (MRI-0116222) and the Capillary LC-Ion Trap Mass spectrometer (CHE-0234863). Components of this work were conducted in a Shared Instrumentation Facility constructed with support from Research Facilities Improvement Grant C06 RR-16572 from the NCRR/NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Li, Q., Chang, YT. A protocol for preparing, characterizing and using three RNA-specific, live cell imaging probes: E36, E144 and F22. Nat Protoc 1, 2922–2932 (2006). https://doi.org/10.1038/nprot.2006.484
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.484
This article is cited by
-
The function-oriented precursor selection for the preparation of carbon dots
Nano Research (2023)
-
Microwave synthesis of 2-[(E)-2-(1H-indol-3-yl)vinyl]hetarenes
Chemistry of Heterocyclic Compounds (2015)
-
In situ simultaneous monitoring of ATP and GTP using a graphene oxide nanosheet–based sensing platform in living cells
Nature Protocols (2014)
-
High-content live cell imaging with RNA probes: advancements in high-throughput antimalarial drug discovery
BMC Cell Biology (2009)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.