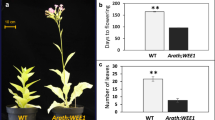
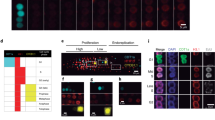
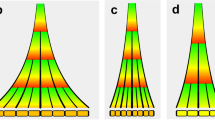
Abstract
Synchronization is a powerful technique for understanding cell cycle events. Here, we describe the procedure for synchronizing tobacco bright yellow 2 (BY-2) cell line, with which an exceptionally high level of synchrony can be achieved. It basically relies on an “arrest-and-release” strategy using aphidicolin, an inhibitor of DNA replication, and propyzamide, a plant-microtubule disruptant. In a single-step process using aphidicolin alone, a cell population with about 70% of the cells at mitosis can be achieved, whereas by a two-step method using the two inhibitors sequentially, the level of synchrony can reach over 90%. The method of choice depends not only on the peak mitotic cell proportion but also on the cell cycle stage that is targeted for analysis. Both procedures take about 1.5 days, and cell cycle progression can be observed from the S phase to the next G1 phase at about 12 h after a 24 h-period treatment with aphidicolin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Uzbekov, R.E. Analysis of the cell cycle and a method employing synchronized cells for study of protein expression at various stages of the cell cycle. Biochemistry 69, 485–496 (2004).
Robbins, E. & Marcus, P.I. Mitotically synchronized mammalian cells: a simple method for obtaining large populations. Science 144, 1152–1153 (1964).
Walker, G.M. Synchronization of yeast cell populations. Methods Cell Sci. 21, 87–93 (1999).
Spudich, J.L. & Sager, R. Regulation of the Chlamydomonas cell cycle by light and dark. J. Cell Biol. 85, 136–145 (1980).
Nagata, T. When I encountered tobacco BY-2 cells! in Tobacco BY-2 cells: Biotech. Agricul. Forest Vol 53 (eds. Nagata, T., Hasezawa, S. & Inze, D.) 1–6 (Springer-Verlag, Berlin, Heidelberg, New York, 2004).
Kato, K. et al. Liquid suspension culture of tobacco cells. Proc. IV IFS: Ferment. Technol. Today 689–695 (1972).
Nagata, T., Nemoto, Y. & Hasezawa, S. Tobacco BY-2 cell line as the “HeLa” cells in the cell biology of higher plants. Int. Rev. Cytol. 132, 1–30 (1992).
Nagata, T. & Kumagai, F. Plant cell biology through the window of the highly synchronized tobacco BY-2 cell line. Methods Cell Sci. 21, 123–127 (1999).
Nagata, T., Okada, K. & Takebe, I. Mitotic protoplasts and their infection with tobacco mosaic virus RNA encapsulated in liposomes. Plant Cell Rep. 1, 250–252 (1982).
Menges, M. & Murray, J.A. Synchronous Arabidopsis suspension cultures for analysis of cell-cycle gene activity. Plant J. 30, 203–212 (2002).
Kakimoto, T. & Shibaoka, H. Cytoskeletal ultrastructure of phragmoplast–nuclei complexes isolated from cultured tobacco cells. Protoplasma Suppl. 2, 95–103 (1988).
Sano, T., Kuraya, Y., Amino, S. & Nagata, T. Phosphate as a limiting factor for the cell division of tobacco BY-2 cells. Plant Cell Physiol. 40, 1–8 (1999).
Ishida, S., Takahashi, Y. & Nagata, T. Isolation of cDNA of an auxin-regulated gene encoding a G protein beta subunit-like protein from tobacco BY-2 cells. Proc. Natl. Acad. Sci. USA 90, 11152–11156 (1993).
Ehsan, H. et al. Effect of indomethacin on cell cycle dependent cyclic AMP fluxes in tobacco BY-2 cells. FEBS Lett. 422, 165–169 (1998).
Laureys, F. et al. A low content in zeatin type cytokinins is not restrictive for the occurrence of G1/S transition in tobacco BY-2 cells. FEBS Lett. 460, 123–128 (1999).
Świątek, A., Lenjou, M., Van Bockstaele, D., Inzé, D. & Van Onckelen, H. Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol. 128, 201–211 (2002).
Sano, T. et al. Calcium ions are involved in the delay of plant cell cycle progression by abiotic stresses. FEBS Lett. 580, 597–602 (2006).
Setiady, Y.Y. et al. Tobacco mitotic cyclins: cloning, characterization, gene expression and functional assay. Plant J. 8, 949–957 (1995).
Ito, M. et al. A novel cis-acting element in promoters of plant B-type cyclin genes activates M phase-specific transcription. Plant Cell 10, 331–341 (1998).
Sorrell, D.A., Combettes, B., Chaubet-Gigot, N., Gigot, C. & Murray, J.A.H. Distinct cyclin D genes show mitotic accumulation or constant levels of transcripts in tobacco bright yellow-2 cells. Plant Physiol. 119, 343–352 (1999).
Combettes, B. et al. Study of phase-specific gene expression in synchronized tobacco cells. Methods Cell Sci. 21, 109–121 (1999).
Reichheld, J.P., Sonobe, S., Clément, B., Chaubet, N. & Gigot, C Cell cycle-regulated histone gene expression in synchronized plant cells. Plant J. 7, 245–252 (1995).
Sekine, M. et al. Isolation and characterization of the E2F-like gene in plants. FEBS Lett. 460, 117–122 (1999).
Hasezawa, S. & Kumagai, F. Dynamic changes and the role of the cytoskeleton during the cell cycle in higher plant cells. Int. Rev. Cytol. 214, 161–191 (2002).
Mayo, K.J., Gonzales, B.J. & Mason, H.S. Genetic transformation of tobacco NT1 cells with Agrobacterium tumefaciens. Nat. Protocols 1, 1105–1111 (2006).
Kumagai, F. et al. Fate of nascent microtubules organized at the M/G1 interface, as visualized by synchronized tobacco BY-2 cells stably expressing GFP-tubulin: time-sequence observations of the reorganization of cortical microtubules in living plant cells. Plant Cell Physiol. 42, 723–732 (2001).
Kutsuna, N., Kumagai, F., Sato, M.H. & Hasezawa, S. Three-dimensional reconstruction of tubular structure of vacuolar membrane throughout mitosis in living tobacco cells. Plant Cell Physiol. 44, 1045–1054 (2003).
Sano, T., Higaki, T., Oda, Y., Hayashi, T. & Hasezawa, S. Appearance of actin microfilament “twin-peaks” in mitosis and their function in cell plate formation, as visualized in tobacco BY-2 cells expressing GFP-fimbrin. Plant J. 44, 595–605 (2005).
Galbraith, D.W. et al. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220, 1049–1051 (1983).
Linsmaier, E.M. & Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant 18, 100–127 (1964).
Planchais, S. et al. Roscovitine, a novel cyclin-dependent kinase inhibitor, characterizes restriction point and G2/M transition in tobacco BY-2 cell suspension. Plant J. 12, 191–202 (1997).
Herbert, R.J. et al. Ethylene induces cell death at particular phases of the cell cycle in the tobacco TBY-2 cell line. J. Exp. Bot. 52, 1615–1623 (2001).
Porceddu, A. et al. A plant-specific cyclin-dependent kinase is involved in the control of G2/M progression in plants. J. Biol. Chem. 276, 36354–36360 (2001).
Miyake, T., Hasezawa, S. & Nagata, T. Role of cytoskeletal components in the migration of nuclei during the cell cycle transition from G1 phase to S phase of tobacco BY-2 cells. J. Plant Physiol. 150, 528–536 (1997).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kumagai-Sano, F., Hayashi, T., Sano, T. et al. Cell cycle synchronization of tobacco BY-2 cells. Nat Protoc 1, 2621–2627 (2006). https://doi.org/10.1038/nprot.2006.381
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.381
This article is cited by
-
Generation of stable transgenic Brassica napus cv. Jet Neuf cell cultures as a tool to investigate in planta protein function
Plant Cell, Tissue and Organ Culture (PCTOC) (2023)
-
Sulfadiazine and phosphinothricin selection systems optimised for the transformation of tobacco BY-2 cells
Plant Cell Reports (2023)
-
Cell cycle synchronization in Panax ginseng roots for cytogenomics research
Horticulture, Environment, and Biotechnology (2022)
-
Flow cytometry and morphometry of tobacco cells expressing the C-terminal domain of the clathrin heavy chain
Plant Cell, Tissue and Organ Culture (PCTOC) (2022)
-
A flow cytometry-based analysis to establish a cell cycle synchronization protocol for Saccharum spp.
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.