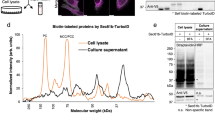
Abstract
Targeted delivery of bioactive molecules to diseased organs or tissues by means of binding molecules specific to markers of diseases represents a promising area of pharmaceutical intervention. The availability of markers of pathology, ideally accessible from the vasculature, is crucial for such strategies. To this aim, here we present a protocol based on terminal perfusion of mice with a reactive ester derivate of biotin that enables the covalent modification of proteins readily accessible from the bloodstream. Biotinylated proteins from total organ or tissue extracts are (i) purified on streptavidin resin in the presence of strong detergents, (ii) digested on the resin and (iii) subjected to proteomic analysis. This technology is applicable to comparative proteomic investigations of differentially expressed, accessible proteins in numerous animal models having different physiological and pathological processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Neri, D. & Bicknell, R. Tumour vascular targeting. Nat. Rev. Cancer 5, 436–446 (2005).
Huang, X. et al. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science 275, 547–550 (1997).
Wu, A.M. & Senter, P.D. Arming antibodies: prospects and challenges for immunoconjugates. Nat. Biotechnol. 23, 1137–1146 (2005).
Adams, G.P. & Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 23, 1147–1157 (2005).
Carter, P. Improving the efficacy of antibody-based cancer therapies. Nat. Rev. Cancer 1, 118–129 (2001).
Winter, G., Griffiths, A.D., Hawkins, R.E. & Hoogenboom, H.R. Making antibodies by phage display technology. Annu. Rev. Immunol. 12, 433–455 (1994).
Silacci, M. et al. Design, construction, and characterization of a large synthetic human antibody phage display library. Proteomics 5, 2340–2350 (2005).
Collins, J., Horn, N., Wadenback, J. & Szardenings, M. Cosmix-plexing: a novel recombinatorial approach for evolutionary selection from combinatorial libraries. J. Biotechnol. 74, 317–338 (2001).
Brody, E.N. & Gold, L. Aptamers as therapeutic and diagnostic agents. J. Biotechnol. 74, 5–13 (2000).
Erlanson, D.A. et al. In situ assembly of enzyme inhibitors using extended tethering. Nat. Biotechnol. 21, 308–314 (2003).
Melkko, S., Scheuermann, J., Dumelin, C.E. & Neri, D. Encoded self-assembling chemical libraries. Nat. Biotechnol. 22, 568–574 (2004).
Shuker, S.B., Hajduk, P.J., Meadows, R.P. & Fesik, S.W. Discovering high-affinity ligands for proteins: SAR by NMR. Science 274, 1531–1534 (1996).
St. Croix, B. et al. Genes expressed in human tumor endothelium. Science 289, 1197–1202 (2000).
Huminiecki, L. & Bicknell, R. In silico cloning of novel endothelial-specific genes. Genome Res. 10, 1796–1806 (2000).
Pasqualini, R. & Ruoslahti, E. Organ targeting in vivo using phage display peptide libraries. Nature 380, 364–366 (1996).
Arap, W., Pasqualini, R. & Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279, 377–380 (1998).
Oh, P. et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature 429, 629–635 (2004).
Rybak, J.N. et al. In vivo protein biotinylation for identification of organ-specific antigens accessible from the vasculature. Nat. Methods 2, 291–298 (2005).
Rybak, J.N., Scheurer, S.B., Neri, D. & Elia, G. Purification of biotinylated proteins on streptavidin resin: a protocol for quantitative elution. Proteomics 4, 2296–2299 (2004).
Zardi, L. et al. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 6, 2337–2342 (1987).
Pini, A. et al. Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J. Biol. Chem. 273, 21769–21776 (1998).
Castellani, P. et al. Differentiation between high- and low-grade astrocytoma using a human recombinant antibody to the extra domain-B of fibronectin. Am. J. Pathol. 161, 1695–1700 (2002).
Borsi, L. et al. Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int. J. Cancer 102, 75–85 (2002).
Berndorff, D. et al. Radioimmunotherapy of solid tumors by targeting extra domain B fibronectin: identification of the best-suited radioimmunoconjugate. Clin. Cancer Res. 11, 7053s–7063s (2005).
Carnemolla, B. et al. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood 99, 1659–1665 (2002).
Ebbinghaus, C. et al. Engineered vascular-targeting antibody-interferon-gamma fusion protein for cancer therapy. Int. J. Cancer 116, 304–313 (2005).
Menrad, A. & Menssen, H.D. ED-B fibronectin as a target for antibody-based cancer treatments. Expert Opin. Ther. Targets 9, 491–500 (2005).
Acknowledgements
We are grateful to the Functional Genomics Center Zurich for access to mass spectrometers and support. Financial support from the EU project STROMA, the Bundesamt für Bildung und Wissenschaft (FLUOR-MMPI and STROMA), the Swiss National Science Foundation and the Gebert-Rüf Foundation are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
The study was designed and the paper written by D.N., C.R. and J.-N.R.; experiments were performed by J.-N.R. and C.R.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Roesli, C., Neri, D. & Rybak, JN. In vivo protein biotinylation and sample preparation for the proteomic identification of organ- and disease-specific antigens accessible from the vasculature. Nat Protoc 1, 192–199 (2006). https://doi.org/10.1038/nprot.2006.29
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.29
This article is cited by
-
Capture and Analysis of Cell Surface N-Glycans by Hydrazide-Modified Magnetic Beads and CE-LIF
Chromatographia (2019)
-
An atlas of bloodstream-accessible bone marrow proteins for site-directed therapy of acute myeloid leukemia
Leukemia (2018)
-
Innovative methods for biomarker discovery in the evaluation and development of cancer precision therapies
Cancer and Metastasis Reviews (2018)
-
Quantity and accessibility for specific targeting of receptors in tumours
Scientific Reports (2014)
-
Differential axial localization along the mouse brain vascular tree of luminal sodium-dependent glutamine transporters Snat1 and Snat3
Journal of Cerebral Blood Flow & Metabolism (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.