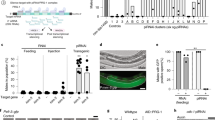
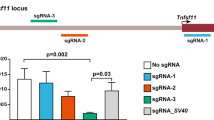
Abstract
RNA interference (RNAi) is an efficient method for silencing genes in cultured cells. Here we describe a simple RNAi approach for silencing genes in a cell type–specific and tissue-specific way in vivo. The approach, which mimics the means by which naturally occurring 'microRNA's are generated, uses a tissue-specific polymerase II promoter to drive the expression of a short hairpin RNA (shRNA) directed against the gene target. The shRNA is cleaved by ubiquitously expressed endonucleases to form an active small interfering RNA of about 22 nt. As a proof of principle, it has been shown that expression of a shRNA directed against the transcription factor Wilms tumor 1 in transgenic mice reduces that protein specifically in nurse cells in the testis. Our transgenic RNAi approach offers a cost-effective means of rapidly (within months) addressing the function(s) of genes of interest in a wide variety of specific cell types and tissues in mice in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nagy, A., Gertsenstein, M.V., Vintersten, K. & Behringer, R. In Manipulating the Mouse Embryo: A Laboratory Manual Vol. 1 (eds. Nagy, A., Gertsenstein, M. V., Vintersten, K. & Behringer, R.) 141–161 (Cold Spring Harbor Laboratory Press, New York, 2002).
Le, Y. & Sauer, B. Conditional gene knockout using cre recombinase. Methods Mol. Biol. 136, 477–485 (2000).
Hamilton, A.J. & Baulcombe, D.C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286, 950–952 (1999).
Hammond, S.M., Bernstein, E., Beach, D. & Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404, 293–296 (2000).
Kunath, T. et al. Transgenic RNA interference in ES cell-derived embryos recapitulates a genetic null phenotype. Nat. Biotechnol. 21, 559–561 (2003).
Hasuwa, H., Kaseda, K., Einarsdottir, T. & Okabe, M. Small interfering RNA and gene silencing in transgenic mice and rats. FEBS Lett. 532, 227–230 (2002).
Peng, S., York, J.P. & Zhang, P. A transgenic approach for RNA interference-based genetic screening in mice. Proc. Natl. Acad. Sci. USA 103, 2252–2256 (2006).
Elbashir, S.M., Lendeckel, W. & Tuschl, T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15, 188–200 (2001).
Stein, P., Svoboda, P. & Schultz, R.M. Transgenic RNAi in mouse oocytes: a simple and fast approach to study gene function. Dev. Biol. 256, 187–193 (2003).
Ventura, A. et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc. Natl. Acad. Sci. USA 101, 10380–10385 (2004).
Tiscornia, G., Tergaonkar, V., Galimi, F. & Verma, I.M. CRE recombinase-inducible RNA interference mediated by lentiviral vectors. Proc. Natl. Acad. Sci. USA 101, 7347–7351 (2004).
Coumoul, X., Shukla, V., Li, C., Wang, R.H. & Deng, C.X. Conditional knockdown of Fgfr2 in mice using Cre-LoxP induced RNA interference. Nucleic Acids Res. 33, e102 (2005).
Lee, R.C. & Ambros, V. An extensive class of small RNAs in Caenorhabditis elegans. Science 294, 862–864 (2001).
Zeng, Y. & Cullen, B.R. Structural requirements for pre-microRNA binding and nuclear export by Exportin 5. Nucleic Acids Res. 32, 4776–4785 (2004).
Zeng, Y. & Cullen, B.R. Sequence requirements for micro RNA processing and function in human cells. RNA 9, 112–123 (2003).
Lee, N.S. et al. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat. Biotechnol. 20, 500–505 (2002).
Rao, M.K. et al. Tissue-specific RNAi reveals that WT1 expression in nurse cells controls germ cell survival and spermatogenesis. Genes Dev. 20, 147–152 (2006).
Xia, X.G., Zhou, H., Samper, E., Melov, S. & Xu, Z. Pol II-expressed shRNA knocks down Sod2 gene expression and causes phenotypes of the gene knockout in mice. PLoS Genet. 2, e10 (2006).
Dickins, R.A. et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet. 37, 1289–1295 (2005).
Chen, X., Wu, J.M., Hornischer, K., Kel, A. & Wingender, E. TiProD: the tissue-specific promoter database. Nucleic Acids Res. 34, D104–D107 (2006).
Reynolds, A. et al. Rational siRNA design for RNA interference. Nat. Biotechnol. 22, 326–330 (2004).
Schwarz, D.S. et al. Asymmetry in the assembly of the RNAi enzyme complex. Cell 115, 199–208 (2003).
Sambrook, J.R. & Russell, D.W. In Molecular Cloning: A Laboratory Manual Vol. 1 (ed. Irwin, N.) 1.84–1.87 (Cold Spring Harbor Laboratory Press, New York, 2001).
Sambrook, J.R. & Russell, D.W. In Molecular Cloning: A Laboratory Manual Vol. 1 (ed. Irwin, N.) 1.90–1.126 (Cold Spring Harbor Laboratory Press, New York, 2001).
Zhou, H., Xia, X.G. & Xu, Z. An RNA polymerase II construct synthesizes short-hairpin RNA with a quantitative indicator and mediates highly efficient RNAi. Nucleic Acids Res. 33, e62 (2005).
Qian, L. et al. T cell receptor-β mRNA splicing: regulation of unusual splicing intermediates. Mol. Cell. Biol. 13, 1686–1696 (1993).
Rao, M.K., Wayne, C.M., Meistrich, M.L. & Wilkinson, M.F. Pem homeobox gene promoter sequences that direct transcription in a Sertoli cell-specific, stage-specific, and androgen-dependent manner in the testis in vivo. Mol. Endocrinol. 17, 223–233 (2003).
Wang, J. & Wilkinson, M.F. Site-directed mutagenesis of large (13-kb) plasmids in a single-PCR procedure. Biotechniques 29, 976–978 (2000).
Maclean, J.A., II et al. Rhox: a new homeobox gene cluster. Cell 120, 369–382 (2005).
Zeng, Y., Yi, R. & Cullen, B.R. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc. Natl. Acad. Sci. USA 100, 9779–9784 (2003).
Southern, J. Southern blotting. Nat. Protoc. 1, 518–525 (2006).
Gudikote, J.P. & Wilkinson, M.F. T-cell receptor sequences that elicit strong down-regulation of premature termination codon-bearing transcripts. EMBO J. 21, 125–134 (2002).
Miyagishi, M. & Taira, K. U6 promoter-driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat. Biotechnol. 20, 497–500 (2002).
Acknowledgements
The authors thank John Pham for technical assistance. This work was supported by National Institutes of Health grants GM58595 and HD042714.
Author information
Authors and Affiliations
Contributions
M.K.R. designed and did the experiments and prepared the manuscript; M.F.W. prepared the manuscript and provided grant support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rao, M., Wilkinson, M. Tissue-specific and cell type–specific RNA interference in vivo. Nat Protoc 1, 1494–1501 (2006). https://doi.org/10.1038/nprot.2006.260
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.260
This article is cited by
-
Defective Wnt3 expression by testicular Sertoli cells compromise male fertility
Cell and Tissue Research (2018)
-
NF-κB-mediated degradation of the coactivator RIP140 regulates inflammatory responses and contributes to endotoxin tolerance
Nature Immunology (2012)
-
Gene therapy with RNAi targeting UHRF1 driven by tumor-specific promoter inhibits tumor growth and enhances the sensitivity of chemotherapeutic drug in breast cancer in vitro and in vivo
Cancer Chemotherapy and Pharmacology (2012)
-
Neuroblastoma-specific expression of potential therapeutics cannot be achieved using a promoter region of the NCX (TLX2) gene
Cancer Gene Therapy (2010)
-
Tumor-specific RNAi targeting eIF4E suppresses tumor growth, induces apoptosis and enhances cisplatin cytotoxicity in human breast carcinoma cells
Breast Cancer Research and Treatment (2009)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.