Abstract
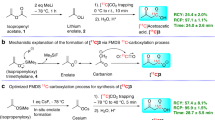
Here we present a protocol for labeling aliphatic carboxylic acids with the positron-emitting radionuclide 11C (t1/2 = 20.4 min) at the carboxyl position using [11C]carbon monoxide via photoinitiated free radical-mediated carbonylation. A solution of an alkyl iodide in a homogenous binary organic solvent–water mixture is introduced into a high-pressure photochemical reactor containing [11C]carbon monoxide. Then the reactor contents are pressurized to 40 MPa and irradiated with ultraviolet light for 6 min. The labeled product is purified using HPLC. All manipulations with radioactivity including the labeling synthesis are carried out on an automated Synthia system. In a typical case, 3.19 GBq of purified [1-11C]1,10-decanedicarboxylic acid (with a specific radioactivity of 188 GBq/μmol) can be obtained within 35 min after the end of a 10-μAh bombardment. Compared to previous labeling methods, this protocol is compatible with a wider range of functional groups, utilizes less-sensitive precursors, and is less subject to isotopic dilution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Positron Emission Tomography: Basic Science and Clinical Practice (eds. Bailey, B.L., Townsend, D.W., Valk, P.E., Maisey, M.N.) (Springer-Verlag, London, 2003).
PET for Drug Development and Evaluation (ed. Comar, D.) (Kluwer Academic Publishers, Dordrecht, Netherlands, 1995).
Halldin, C., Gulyas, B. & Farde, L. PET studies with carbon-11 radioligands in neuropsychopharmacological drug development. Curr. Pharm. Des. 7, 1907–1929 (2001).
Kawashima, H., Yajima, K., Kuge, Y., Hashimoto, N. & Miyake, Y. Synthesis of [1-11C]-2-octynoic acid, [1-11C]-2-decynoic acid and [1-11C]-3-(R,S)-methyloctanoic acid as potential markers for PET studies of fatty acid metabolism. J. Label. Compd. Radiopharm. 39, 181–193 (1997).
Antoni, G. & Långström, B. Synthesis of g-amino[4-11C]butyric acid (GABA). J. Label. Compd. Radiopharm. 27, 571–576 (1989).
Chatgilialoglu, C., Crich, D., Komatsu, M. & Ryu, I. Chemistry of acyl radicals. Chem. Rev. 99, 1991–2070 (1999).
Kihlberg, T. & Långström, B. Biologically active 11C-labeled amides using palladium-mediated reactions with aryl halides and [11C]carbon monoxide. J. Org. Chem. 64, 9201–9205 (1999).
Karimi, F. & Långström, B. Palladium-mediated carboxylation of aryl halides (triflates) or benzyl halides using [13C]/[11C]carbon monoxide with tetramethylammonium hydroxide or trimethylphenylammonium hydroxide. J. Chem. Soc., Perkin Trans. 1 20, 2256–2259 (2002).
Doi, H. et al. Synthesis of 11C-labeled N,N′-diphenylurea and ethyl phenylcarbamate by a rhodium-promoted carbonylation via [11C]isocyanatobenzene using phenyl azide and [11C]carbon monoxide. Org. Biomol. Chem. 2, 3063–3066 (2004).
Ryu, I. Radical carboxylations of iodoalkanes and saturated alcohols using carbon monoxide. Chem. Soc. Rev. 30, 16–25 (2001).
Ryu, I., Sonoda, N. & Curran, D.P. Tandem radical reactions of carbon monoxide, isonitriles, and other reagent equivalents of the geminal radical acceptor/radical precursor synthon. Chem. Rev. 96, 177–194 (1996).
Itsenko, O., Kihlberg, T. & Långström, B. Carboxylation of alkyl iodides with [11C] and (13C)carbon monoxide: Using sulfoxides as oxygen nucleophiles. Synlett 20, 3154–3156 (2005).
Itsenko, O., Kihlberg, T. & Långström, B. Synthesis of aliphatic [carbonyl-11C]esters using [11C]carbon monoxide. Eur. J. Org. Chem. 3830–3834 (2005).
Itsenko, O., Kihlberg, T. & Långström, B. Photoinitiated carbonylation with [11C]carbon monoxide using amines and alkyl iodides. J. Org. Chem. 69, 4356–4360 (2004).
Kihlberg, T., Itsenko, O., Ferm, T. & Långström, B. Method and apparatus for the use of [11C] carbon monoxide in synthesis of carboxamides for positron emission tomography by photoinitiated carbonylation. PCT Int. Appl. WO2005042441, 2005. Chem. Abstr. 142, 463193 (2005).
Bjurling, P., Reineck, R., Westerberg, G., Gee, A.D., Sutcliffe, J. & Långström, B. in Proceedings of the VIth Workshop on Targetry and Target Chemistry 282–284 (TRIUMF, Vancouver, Canada, 1995).
Itsenko, O. & Langstrom, B. Photoinitiated free radical carbonylation enhanced by photosensitizers. Org. Lett. 7, 4661–4664 (2005).
Acknowledgements
We thank T. Ferm for his skillful technical assistance. Financial support from the Swedish Research Council and Uppsala University Amersham Fund is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Itsenko, O., Kihlberg, T. & Långström, B. Labeling of aliphatic carboxylic acids using [11C]carbon monoxide. Nat Protoc 1, 798–802 (2006). https://doi.org/10.1038/nprot.2006.112
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.112
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.