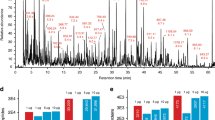
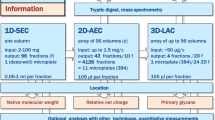
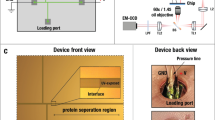
Abstract
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) is the most popular and versatile method of protein separation among a rapidly growing array of proteomics technologies. Based on two distinct procedures, it combines isoelectric focusing (IEF), which separates proteins according to their isoelectric point (pI), and SDS-PAGE, which separates them further according to their molecular mass. At present, 2D-PAGE is capable of simultaneously detecting and quantifying up to several thousand protein spots in the same gel image. Here we provide comprehensive step-by-step instructions for the application of a standardized 2D-PAGE protocol to a sample of human plasma or cerebrospinal fluid (CSF). The method can be easily adapted to any type of sample. This four-day protocol provides detailed information on how to apply complex biological fluids to an immobilized dry strip gel, cast home-made gradient acrylamide gels, run the gels, and perform standard staining methods. A troubleshooting guide is also included.
NOTE:The version of this article initially published online contained the following errors in the REAGENT SETUP section on p. 814: Under “Strip reswelling buffer”, “0.0375 g of DTE” should be “0.0385 g of DTE”. Under “Iso Buffer”, “5.4 g of urea” should be “4.8 g of urea”. Under “Electrophoresis buffer, upper tank”, “18 g of Tris” should be “9.09 g of Tris” and “86.4 g of glycine” should be “44.6 g of glycine”. Under “Electrophoresis buffer, lower tank”, “90 g of Tris” should be “45.45 g of Tris” and “432 g of glycine” should be “222.9 g of glycine”. Under “Agarose sealing solution”, “5%” should be “0.5%”. Under “Sensitizer solution”, “68.4 g of sodium acetate” should be “68.04 g of sodium acetate trihydrate”. Under “Ammoniacal silver nitrate solution”, “Silver nitrate” should be “47 mM silver nitrate”; “10 N sodium hydroxide” should be “0.02 N sodium hydroxide”; and “25% ammonium hydroxide (27% pure, Merck)” should be 0.33% ammonium hydroxide (25% pure, Merck)”. The sentence “Add 6 g of silver nitrate dissolved in dH2O to 30ml slowly into a solution containing 160 ml of dH2O, 10 ml of concentrated ammonia (25%) and 1.5 ml of 10 N sodium hydroxide” should read “Dissolve 6 g of silver nitrate in dH2O to 30 ml, then add slowly into a solution containing 160 ml of dH2O, 10 ml of 25% ammonium hydroxide and 1.5 ml of 10 N sodium hydroxide.” In Table 1 (p. 816), “Up to 100,000 kVh” should be “100kVh”. These errors have been corrected in all versions of the article.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
22 March 2007
see erratum
References
O'Farrell, P.H. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250, 4007–4721 (1975).
Klose, J. Protein mapping by combined isoelectric focusing and electrophoresis of mouse tissues. A novel approach to testing for induced point mutations in mammals. Humangenetik 26, 231–243 (1975).
Klose, J. & Kobalz, U. Two-dimensional electrophoresis of proteins: an updated protocol and implications for a functional analysis of the genome. Electrophoresis 16, 1034–1059 (1995).
Bjellqvist, B. et al. Isoelectric focusing in immobilized pH gradients: principle, methodology and some applications. J. Biochem. Biophys. Methods 6, 317–339 (1982).
Rabilloud, T., Adessi, C., Giraudel, A. & Lunardi, J. Improvement of the solubilization of proteins in two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 18, 307–316 (1997).
Sanchez, J.C., Hochstrasser, D. & Rabilloud, T. In-gel sample rehydration of immobilized pH gradient. Methods Mol. Biol. 112, 221–225 (1999).
Deshusses, J.M. et al. Exploitation of specific properties of trifluoroethanol for extraction and separation of membrane proteins. Proteomics 3, 1418–1424 (2003).
Bjellqvist, B., Pasquali, C., Ravier, F., Sanchez, J.C. & Hochstrasser, D. A nonlinear wide-range immobilized pH gradient for two-dimensional electrophoresis and its definition in a relevant pH scale. Electrophoresis 14, 1357–1365 (1993).
Gorg, A., Postel, W. & Gunther, S. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 9, 531–546 (1988).
Yan, J.X., Sanchez, J.C., Rouge, V., Williams, K.L. & Hochstrasser, D.F. Modified immobilized pH gradient gel strip equilibration procedure in SWISS-2DPAGE protocols. Electrophoresis 20, 723–726 (1999).
Hochstrasser, D.F., Patchornik, A. & Merril, C.R. Development of polyacrylamide gels that improve the separation of proteins and their detection by silver staining. Anal. Biochem. 173, 412–423 (1988).
Hochstrasser, D.F. & Merril, C.R. 'Catalysts' for polyacrylamide gel polymerization and detection of proteins by silver staining. Appl. Theor. Electrophor. 1, 35–40 (1988).
Hochstrasser, D.F., Harrington, M.G., Hochstrasser, A.C., Miller, M.J. & Merril, C.R. Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal Biochem 173, 424–435 (1988).
Görg, A., Weiss, W. & Dunn, M.J. Current two-dimensional electrophoresis technology for proteomics. Proteomics 4, 3665–3685 (2004).
Yan, J.X., Harry, R.A., Spibey, C. & Dunn, M.J. Postelectrophoretic staining of proteins separated by two-dimensional gel electrophoresis using SYPRO dyes. Electrophoresis 21, 3657–3665 (2000).
Unlu, M., Morgan, M.E. & Minden, J.S. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis 18, 2071–2077 (1997).
Righetti, P.G. et al. Critical survey of quantitative proteomics in two-dimensional electrophoretic approaches. J. Chromatogr. A 1051, 3–17 (2004).
Appel, R. et al. Automatic classification of two-dimensional gel electrophoresis pictures by heuristic clustering analysis: a step toward machine learning. Electrophoresis 9, 136–142 (1988).
Wilkins, M.R., Hochstrasser, D.F., Sanchez, J.C., Bairoch, A. & Appel, R.D. Integrating two-dimensional gel databases using the Melanie II software. Trends Biochem. Sci. 21, 496–497 (1996).
Appel, R.D. et al. Melanie II—a third-generation software package for analysis of two-dimensional electrophoresis images. I. Features and user interface. Electrophoresis 18, 2724–2734 (1997).
Patton, W.F., Schulenberg, B. & Steinberg, T.H. Two-dimensional gel electrophoresis; better than a poke in the ICAT? Curr. Opin. Biotechnol. 13, 321–328 (2002).
Wu, W.W., Wang, G., Baek, S.J. & Shen, R.F. Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel- or LC-MALDI TOF/TOF. J. Proteome Res. 5, 651–658 (2006).
Shaw, M.M. & Riederer, B.M. Sample preparation for two-dimensional gel electrophoresis. Proteomics 3, 1408–1417 (2003).
Ahmed, N. & Rice, G.E. Strategies for revealing lower abundance proteins in two-dimensional protein maps. J. Chromatogr. B 815, 39–50 (2005).
Banks, R.E. et al. The potential use of laser capture microdissection to selectively obtain distinct populations of cells for proteomic analysis—preliminary findings. Electrophoresis 20, 689–700 (1999).
Lescuyer, P., Hochstrasser, D.F. & Sanchez, J.C. Comprehensive proteome analysis by chromatographic protein prefractionation. Electrophoresis 25, 1125–1135 (2004).
Righetti, P.G., Castagna, A., Antonioli, P. & Boschetti, E. Prefractionation techniques in proteome analysis: the mining tools of the third millennium. Electrophoresis 26, 297–319 (2005).
Sapan, C.V., Lundblad, R.L. & Price, N.C. Colorimetric protein assay techniques. Biotechnol. Appl. Biochem. 29, 99–108 (1999).
Hochstrasser, D. et al. 'High-resolution' mini-two-dimensional gel electrophoresis automatically run and stained in less than 6 h with small, ready-to-use slab gels. Clin. Chem. 34, 166–170 (1988).
Rabilloud, T. (ed.) Proteome Research: Two-dimensional Gel Electrophoresis and Identification Methods (Principles and Practice) (Springer, Berlin, 2000).
Westermeier, R. & Naven, T. Proteomics in Practice (John Wiley & Sons, Weinheim, Germany, 2002).
Link, A.J. (ed.) 2-D Proteome Analysis Protocols (Humana Press, Totowa, NJ, 1999).
Hoogland, C., Mostaguir, K., Sanchez, J.C., Hochstrasser, D.F. & Appel, R.D. SWISS-2DPAGE, ten years later. Proteomics 4, 2352–2356 (2004).
Acknowledgements
Many colleagues have contributed to the development of this protocol including B. Bjellqvist, V. Converset, M. Côte, O. Golaz, C. Pasquali, F. Ravier, N. Rodrigo, L. Tonella and C. Zimmermann.
Author information
Authors and Affiliations
Contributions
O.C. prepared the manuscript and optimized SyproRuby fluorescent staining; P.R.B. optimized 2D-PAGE of CSF samples; J.-C.S. and D.F.H. developed the 2D-PAGE protocol.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Carrette, O., Burkhard, P., Sanchez, JC. et al. State-of-the-art two-dimensional gel electrophoresis: a key tool of proteomics research. Nat Protoc 1, 812–823 (2006). https://doi.org/10.1038/nprot.2006.104
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.104
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.