Abstract
Early-life conditions can contribute to the propensity for developing neuropsychiatric disease, including substance abuse disorders. However, the long-lasting mechanisms that shape risk or resilience for drug addiction remain unclear. Previous work has shown that a neonatal handling procedure in rats (which promotes enriched maternal care) attenuates morphine conditioning, reduces morphine-induced glial activation, and increases microglial expression of the anti-inflammatory cytokine interleukin-10 (IL-10). We thus hypothesized that anti-inflammatory signaling may underlie the effects of early-life experience on later-life opioid drug-taking. Here we demonstrate that neonatal handling attenuates intravenous self-administration of the opioid remifentanil in a drug-concentration-dependent manner. Transcriptional profiling of the nucleus accumbens (NAc) from handled rats following repeated exposure to remifentanil reveals a suppression of pro-inflammatory cytokine and chemokine gene expression, consistent with an anti-inflammatory phenotype. To determine if anti-inflammatory signaling alters drug-taking behavior, we administered intracranial injections of plasmid DNA encoding IL-10 (pDNA-IL-10) into the NAc of non-handled rats. We discovered that pDNA-IL-10 treatment reduces remifentanil self-administration in a drug-concentration-dependent manner, similar to the effect of handling. In contrast, neither handling nor pDNA-IL-10 treatment alters self-administration of food or sucrose rewards. These collective observations suggest that neuroimmune signaling mechanisms in the NAc are shaped by early-life experience and may modify motivated behaviors for opioid drugs. Moreover, manipulation of the IL-10 signaling pathway represents a novel approach for influencing opioid reinforcement.
Similar content being viewed by others
Introduction
Opioid drugs can be effective pain relievers, but they are also highly addictive substances that can lead to drug dependence and carry a significant risk for overdosing. In the United States, overdose deaths from opioids have risen dramatically over the past decade (Calcaterra et al, 2013; Rudd et al, 2016). Developing solutions to this growing epidemic will be facilitated by a better understanding of the biological and environmental factors that promote vulnerability to, or resilience from, opioid abuse.
Drug addiction is thought to arise from structural and functional adaptations in brain regions associated with motivation and reward, in particular the dopamine-producing neurons of the ventral tegmental area and one of its major targets, the nucleus accumbens (NAc) in the ventral striatum (Robinson and Berridge, 1993). Although the majority of addiction research has focused on identifying the cellular and molecular adaptations of neurons and their associated circuitries (Hyman et al, 2006; Lüscher and Malenka, 2011), there is emerging evidence that pathways of neuroimmune signaling (eg, between neurons and glial cells) play an important role in the neurophysiological and behavioral manifestations of acute and repeated exposure to drugs of abuse (Lacagnina et al, 2017; Miguel-Hidalgo, 2009).
Glial activity in the context of neuroimmune signaling can be broadly categorized as either anti-inflammatory or pro-inflammatory depending on the cytokines and chemokines expressed and the functional outcomes they support (Dantzer et al, 2008; Meyer et al, 2008), although their actions can be complex and multidimensional (Ginhoux et al, 2016; Ransohoff, 2016). Opioid administration produces pro-inflammatory glial activation via Toll-like receptor 4 (TLR4) signaling on microglia and other immunocompetent cells, and the resulting production of cytokines and chemokines may contribute to the reinforcing properties of opioids (Hutchinson et al, 2012; Wang et al, 2012). In contrast, anti-inflammatory neuroimmune signaling has been shown to attenuate the reinforcing effects of opioids, and its expression in the brain can be profoundly influenced by early-life environmental conditions (Schwarz et al, 2011). Specifically, neonatal handling in rats (in which daily, brief separation of pups from the dam promotes increased maternal licking and grooming behavior) (Liu et al, 1997) prevents reinstatement of morphine conditioned place preference in adulthood, reduces morphine-induced pro-inflammatory cytokine and chemokine expression in the NAc, and persistently increases gene expression of the anti-inflammatory cytokine interleukin-10 (IL-10) in microglia (Schwarz et al, 2011). Because IL-10 is a powerful modulator of pro-inflammatory signaling (Kwilasz et al, 2015; Moore et al, 2001), these data raise the possibility that environmental conditions early in life may influence liability for drug addiction via changes in IL-10 in the NAc, although several questions remain. First, it is unclear if the ‘protective’ effect of neonatal handling for opioid conditioned place preference can be extended to a model of opioid self-administration, which more accurately represents some essential features of human drug-taking behaviors (Fernando and Robbins, 2011). Second, the effect of repeated opioid exposure on NAc neuroimmune gene expression is largely unknown. And finally, while changes in IL-10 are associated with handling, it is unknown if direct manipulation of IL-10 is sufficient to influence addiction-related behaviors.
To address these questions, we sought to determine the role of anti-inflammatory signaling, in particular IL-10, in the NAc during self-administration of the opioid remifentanil. Our data demonstrate the following: (1) early-life handling reduces opioid self-administration in adulthood in a drug-concentration-dependent manner, without affecting food or sucrose self-administration; (2) transcriptional profiling of the NAc reveals that neonatal handling persistently increases IL-10 expression and suppresses pro-inflammatory cytokine/chemokine signaling despite repeated opioid exposure; and (3) delivery of IL-10 gene therapy into the NAc of non-handled rats reduces opioid drug-taking without affecting food or sucrose reward, demonstrating specificity to opioid reinforcement. Together, these data implicate NAc anti-inflammatory signaling as an important substrate influencing self-motivated opioid consumption under defined conditions, while providing a novel therapeutic target to exploit in the pursuit of improving treatments for opioid addiction.
Materials and methods
Subjects
Adult male and female Sprague-Dawley rats (males 250–275 g; females 175–199 g; Harlan/Envigo, Dublin, VA) were housed in individually ventilated cages with corn cob bedding and ad libitum access to food (Purina Lab Diet 5001) and water. The colony was maintained at 23 °C on a 12 : 12 h light–dark cycle (lights on at 0700 hours), and cages were changed once per week. Rats were housed in same-sex pairs for 1 week before experimental manipulations. For handling experiments, males and females were paired for breeding; when females showed signs of pregnancy, males were removed from the cage. Litters were culled to two females and up to eight male pups per litter on postnatal day 2 (P2), and only males were used in experiments. For plasmid experiments, adult male rats (250–275 g; Harlan/Envigo, Dublin, VA) were pair-housed and given at least 1 week to acclimate to laboratory conditions. All experiments were approved by the Duke University Institutional Animal Care and Use Committee.
Neonatal Handling
Approximately half of the litters were assigned to a brief separation/neonatal handling condition (‘Handled’) as previously described (Bilbo et al, 2007; Schwarz et al, 2011). For each handled litter, the dam was removed from the home cage and placed in a clean polycarbonate cage with a securely fastened lid, while pups were moved one at a time to the center of a separate, clean polycarbonate cage with bedding. After 15 min, pups were returned to their home cage, followed by the dams. This handling procedure was conducted once per day between 1400 and 1600 hours from P2 to P20; control litters (‘Control’) were left undisturbed during this time. On P22, females were culled while all males were weaned and pair-housed with siblings until they reached adulthood (P60). To reduce litter-specific effects, only two to four pups per litter were used in each experiment (mean number of pups from the same litter used per experiment=2.5±0.14). Adult animals were transferred to the colony room adjacent to behavioral testing rooms and were allowed to acclimate to housing conditions (reverse 12 : 12 light cycle, lights off at 0700 hours) for at least 1 week. All experiments were conducted during the animals’ active phase.
Self-Administration
Behavioral testing was conducted in operant-conditioning chambers (Med Associates, ENV-008-CT). For remifentanil experiments, overnight training to lever-press for food was performed as previously described (Levin et al, 2016). After reaching response criteria (⩾50 responses in 3 consecutive 30-min sessions), rats were implanted with intravenous catheters as previously described (Hall et al, 2015). Briefly, rats were anesthetized with a combination of ketamine (60 mg/kg, i.p.) and dexmedetomidine (0.15 mg/kg, i.p.), the jugular vein was isolated, and a sterile silastic catheter (SAI Infusion Technologies) was inserted and fastened with silk sutures. The opposite end was tunneled subcutaneously to emerge between the scapulae and was attached to an infusion harness (SAI). Rats were given a minimum of 1 week to recover from surgery. Subsequently, rats were allowed to self-administer the opioid remifentanil intravenously on a fixed ratio 1 (FR1) schedule of reinforcement with a 20 s time-out period. Remifentanil was chosen because of its high specificity for μ-opioid receptors and its short half-life, resulting in high rates of responding (Panlilio and Schindler, 2000). Food was temporarily restricted 24 h before the first remifentanil session to motivate initial exploration of the active lever, but was returned to ab libitum access thereafter. Rats received daily 1 h remifentanil sessions for 14 days: 7 days at 0.29 μg/kg and 7 days at 0.9 μg/kg. Rats were saline perfused 14 days after the final session.
For dose–response experiments, animals were allowed to acquire remifentanil at 0.9 μg/kg (1 h daily, FR1, 20 s time-out) without previous food training until responding was stable (defined as <20% variance of infusions received across the previous three sessions, for a minimum of 14 days). Subsequently, rats received daily exposure to one of four concentrations of remifentanil (0.09, 0.29, 0.9, or 2.9 μg/kg) based on previously established doses (Hutchinson et al, 2012) in a randomized sequence. To control for order effects, animals from both groups were counterbalanced to receive each dose sequence permutation, and all animals were exposed to all four doses in 4-day cycles. There were no external stimuli indicating which dose would be delivered each day, and the order of doses received in a 4-day cycle did not influence the order received in a subsequent cycle. The dose–response sessions proceeded for 12 days (1 h daily, FR1, 20 s time-out). Rats were saline perfused 24 h after the final session.
For food and sucrose experiments, rats were either food restricted (to 85–90% of free-feeding body weight) and allowed to lever-press for food pellets (TestDiet, 45 mg), or maintained on ad libitum diet and allowed to lever-press for sucrose pellets (Bio-Serv, 45 mg, banana flavored). Both conditions received daily 1 h sessions, FR1, 20 s time-out. For all self-administration experiments, the number of drug infusions or pellets acquired per session is reported, along with responses on the inactive lever. The ordinate scale for inactive responses is based on matching the scale for active lever responses. See Supplementary Methods for additional information.
Drugs
Remifentanil hydrochloride (Sigma-Aldrich, cat. R1908) was dissolved into 0.9% sterile saline to a concentration of 100 μg/ml and frozen in aliquots at −20 °C, which were thawed and further diluted with 0.9% saline to the required concentration prior to daily self-administration sessions. D-(+)-mannose (Sigma-Aldrich, cat. M6020) was dissolved in 0.9% sterile saline before use.
Tissue Processing
Rats were saline perfused and the NAc was dissected and rapidly frozen for subsequent mRNA or protein analyses (see Supplementary Methods). For in vitro plasmid DNA (pDNA) experiments, cells from whole brains (excluding cerebellum) were cultured either without cell-type-specific isolation or following CD11b microglial isolation as previously described (Williamson et al, 2011). Following pDNA behavioral experiments, tissue was fixed in 4% paraformaldehyde, cryoprotected in 30% sucrose, sectioned (40 μm), thaw-mounted directly to Superfrost Plus slides (VWR), coverslipped with Vectashield (Vector), and imaged to confirm infusion site.
Quantitative Real-Time PCR
RNA was isolated using the TRIzol method (Ambion). For tissue collected from rats used in Figure 1, cDNA was synthesized using the QuantiTect reverse transcription kit (Qiagen) and relative gene expression to Gapdh was measured using the QuantiFast SYBR Green PCR kit (Qiagen). For tissue collected from rats used in Figure 2, cDNA was synthesized using the RT2 First Strand kit (Qiagen) and gene expression was quantified with PCR arrays (SABiosciences/Qiagen; cat. PARN-022ZA) using RT2 SYBR Green ROX master mix (Qiagen). See Supplementary Methods for additional information.
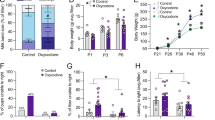
Neonatal handling attenuates remifentanil self-administration in a drug-concentration-dependent manner. (a) Schematic representation of the experimental timeline. Handling was performed by separating dams from their litter for 15 min/day from P2–P20, whereas controls were left undisturbed. Following early-life conditions, adult rats were trained to self-administer intravenous remifentanil (n=11–13/group). (b) The number of intravenous infusions of remifentanil was recorded in daily 1 h sessions (FR1, 20 s time-out) for 14 days: 7 days at 0.29 μg/kg and 7 days at 0.9 μg/kg. (c) Neonatal handling does not influence remifentanil self-administration for adult rats during initial sessions at 0.29 μg/kg when comparing the first and final sessions (day 1 vs day 7). In contrast, neonatal handling significantly attenuates the number of remifentanil infusions acquired on day 7 when remifentanil is delivered at 0.9 μg/kg (*p<0.05). (d) Responses on the inactive lever were recorded across sessions. (e) Neonatal handling has no effect on inactive lever pressing when comparing day 1 vs day 7 for sessions at both drug concentrations.
Neonatal handling lowers dose–response curve of remifentanil self-administration. (a) Schematic representation of the experimental timeline. Following neonatal handling or control conditions, adult rats were allowed to acquire remifentanil self-administration at 0.9 μg/kg (1 h daily, FR1, 20 s time-out) until responding was stable (<20% variance across three sessions) for at least 14 days. Subsequent sessions involved randomized access to varying concentrations of remifentanil, which was counterbalanced between groups (four concentrations, three sessions per concentration, across 12 days; n=6–8/group). (b) There is a downward shift in the dose–response curve to remifentanil in rats that received neonatal handling compared with controls (main effect of early-life experience; *p<0.05). Post hoc tests revealed that handled rats administer fewer remifentanil infusions per session at the 0.09 or 0.29 μg/kg concentrations relative to controls (*p<0.05, **p<0.01). (c) Handled and non-handled control rats do not show differences in responding on the inactive lever during the dose–response sessions.
Gene Functional Annotation Clustering Analysis
To assess the functional biological pathways that characterize neonatal handling following remifentanil, the top 20 genes that were up- or downregulated in the PCR array between control and handled rats were uploaded into DAVID functional gene ontology software (https://david.ncifcrf.gov/). All non-housekeeping genes that were expressed in the array served as ‘background’. Functional annotation was run, and the top 12 enriched annotation groups (functional clusters) were chosen to represent the group comparisons (Huang et al, 2009).
Western Blot
Protein was precipitated from the phenol–ethanol fractions remaining from TRIzol-mediated RNA extraction as described previously (Kopec et al, 2017), and the ratio of phosphorylated to total protein was assessed by western blot analysis (see Supplementary Methods).
pDNA Treatments
The pDNA vectors (pDNA-control or pDNA-IL-10) were prepared as previously described (Milligan et al, 2006). For in vitro pDNA experiments, cultured cells (either dissociated brain tissue or CD11b-isolated cultures) were incubated with D-mannose (5 mg/ml) and pDNA (10 μg/ml) for 2 h. For in vivo pDNA treatments, rats underwent stereotaxic surgery to receive bilateral intracranial injections of pDNA and D-mannose into the NAc (7.5 μg/μl pDNA and 50 μg/μl D-mannose). A Hamilton syringe was lowered to the target coordinates (AP: +1.8; ML: ±1.5; DV: −6.8) (Paxinos and Watson, 2007) and 1 μl of pDNA and D-mannose solution was slowly infused (0.1 μl/min). After at least 1 week of recovery, rats were killed for mRNA and protein analysis, or continued to self-administer for remifentanil or food/sucrose as described above. See Supplementary Methods for additional details.
Statistical Analysis
Data were analyzed and depicted using GraphPad Prism 6 software. Behavioral data were analyzed by two-way repeated measures analysis of variance (RM-ANOVA), with early life experience (Control or Handled) or pDNA treatment (pDNA-control or pDNA-IL-10) as the between-subjects factor, and time as the within-subjects factor. Behavioral analyses (RM-ANOVA and post hoc comparisons) were a priori restricted to the first and final sessions (ie, day 1 vs day 7) for all conditions. Sessions for different remifentanil doses were analyzed separately. Dose–response curve data were analyzed by two-way RM-ANOVA, with early life experience as the between-subjects factor and drug concentration as the within-subjects factor. Outcomes of behavioral data were followed by Fisher’s protected least significant difference post hoc tests when appropriate. Outliers from behavioral assessments were detected by Grubb’s test, resulting in the removal of three subjects (n=1 control, n=2 handled). Normality was tested with D’Agostino-Pearson omnibus normality test. Single-gene PCR data were analyzed by one- or two-way ANOVAs followed by Tukey’s or Bonferroni’s post hoc tests when appropriate, or analyzed by Kruskal–Wallis test followed by Dunn’s post hoc test for non-normally distributed data. PCR array data and figures were generated with the assistance of software provided by the manufacturer (RT2 Profiler PCR Array Data Analysis 3.5, SABiosciences/Qiagen), and fold regulation of gene expression was analyzed by two-tailed Student’s t-test. Western blot and PCR data for in vivo pDNA characterization were analyzed by two-tailed Student’s t-test. The threshold for significance was set at p<0.05. All data are presented as mean±SEM.
Results
Neonatal Handling Attenuates Remifentanil Self-Administration in a Drug-Concentration-Dependent Manner
To test the impact of neonatal handling on a behavioral paradigm of self-motivated opioid drug taking, we measured self-administration behavior for remifentanil at two drug concentrations (0.29 and 0.9 μg/kg) for 7 days each (Figure 1a). Remifentanil infusions acquired per session were measured over time (Figure 1b). A two-way RM-ANOVA comparing the first and final sessions for each drug concentration (Figure 1c) revealed no main effect of early-life experience on the number of infusions acquired at 0.29 μg/kg, and no change in behavior over time. In contrast, there was a main effect of time (F1,22=79.72, p<0.001) and a significant interaction between time and early-life experience (F1,22=4.61, p<0.05) for sessions at 0.9 μg/kg. Post hoc comparison-revealed controls took significantly more 0.9 μg/kg remifentanil infusions on day 7 compared with handled rats (p<0.05; Figure 1c). Inactive lever responses were also compared as a measure of non-specific lever displacement (Figure 1d). Responses on the inactive lever did not differ across time or between groups for sessions at 0.29 μg/kg. There was a significant interaction for sessions at 0.9 μg/kg (F1,22=6.14, p<0.05); however, post hoc comparisons determined there was no difference between groups at either day 1 or day 7 (p>0.05; Figure 1e).
Neonatal Handling Shifts the Dose–Response Function for Remifentanil
These data reveal that neonatal handling influences self-motivated remifentanil consumption, although the response was highly dependent upon drug concentration and emerged on day 7 after the drug concentration was shifted to 0.9 μg/kg. To further investigate the relationship between early-life experience and self-administration behavior as a factor of remifentanil concentration, we trained rats until responding was stable at the 0.9 μg/kg dose and examined the dose–response curve following randomized exposure to four different drug concentrations (Figure 2a). Individual responses for each concentration were averaged across three separate sessions and are depicted as the group mean±SEM (Figure 2b). A two-way RM-ANOVA revealed that neonatal handling significantly reduced the number of drug infusions acquired (main effect of early-life experience; F1,12=4.81, p<0.05; Figure 2b). Post hoc comparisons determined that handled rats acquired fewer remifentanil infusions per session during the 0.09 μg/kg (p<0.01) and 0.29 μg/kg sessions (p<0.05), compared with controls. Interestingly, no group difference was observed for sessions at 0.9 μg/kg, suggesting that the concentration of initial drug exposure and/or number of sessions influenced subsequent drug-taking. The number of infusions acquired was also strongly influenced by drug concentration (main effect of drug concentration; F3,36=27.16, p<0.001), which is consistent with the presumption that rodents modify within-session behavior based on drug received per infusion. Responding on the inactive lever varied by dose (main effect of drug concentration; F3,36=8.09, p<0.001), but we observed no difference between groups for inactive lever pressing (Figure 2c). These results provide evidence that neonatal handling can influence opioid self-administration, and the emergence of this long-lasting effect is strongly dependent upon drug concentration and the sequence of its availability.
Neonatal Handling Produces an Anti-Inflammatory Environment in the NAc Following Remifentanil Exposure
To characterize inflammatory neuroimmune signaling in the NAc, we collected tissue from rats (from Figure 2) and utilized PCR arrays to broadly analyze gene expression of 84 cytokine/chemokine-related genes (Figure 3a). As a baseline drug-naive control group, NAc tissue was also collected from rats that underwent jugular isolation surgery but received no remifentanil (‘Sham’). Transcriptional profiling of the NAc revealed decreased expression of pro-inflammatory cytokine and chemokine genes in handled rats that received remifentanil (ie, Handled-Remi), compared with non-handled controls (ie, Control-Sham); 12 genes investigated were significantly downregulated, including CX3CR1, TLR2, IL-1β, and CCL2 (Figure 3b and Supplementary Table 1), which represent suppression of key messengers and receptors mediating pro-inflammatory responses. Non-supervised hierarchical clustering of all non-housekeeping genes grouped rats primarily by early-life experience (Supplementary Figure S1), indicating a shared organization of neuroimmune gene regulation influenced by handling. Functional annotation clustering analysis of gene enrichment scores from array data comparing handled against control rats after self-administration revealed shared patterns of gene downregulation across a variety of functional clusters related to chemokine signaling and inflammatory regulation (Figure 3c). Although observing gene enrichment for these functional clusters was not surprising (as the gene set was pre-determined from the array), the consistent pattern of neuroimmune gene downregulation in Handled-Remi rats was striking and suggestive of an anti-inflammatory environment in the NAc.
Transcriptional profile of NAc reveals evidence of an anti-inflammatory signature in handled rats. (a) The log2 fold-change plate-layout heat maps of gene expression as assessed by PCR array, compared with Control-Sham (‘Control’=non-handled; ‘Handled’=neonatal handling; ‘Sham’=no remifentanil; ‘Remi’=remifentanil self-administration; n=6/group). (b) Fold regulation and p-values of significant gene expression changes from PCR array (compared with Control-Sham). After prolonged self-administration of remifentanil, Handled-Remi rats in particular show downregulation of a number of immune-related genes (See Supplementary Table 1 for complete gene list). (c) Functional annotation clustering analysis of gene enrichment scores of the top 20 up- or downregulated genes from array data following self-administration (Handled-Remi compared with Control-Remi) demonstrates robust downregulation of gene expression across most functional clusters. (d) TLR4 mRNA is elevated in NAc of rats that self-administered remifentanil (*p<0.05; Sham: n=6/group; Remi: n=11–13/group). (e) Expression of IL-10 mRNA in the NAc is increased for rats that received neonatal handling (*p<0.05; Sham: n=3–4/group; Remi: n=8–10/group).
The anti-inflammatory neuroimmune phenotype in Handled-Remi rats could be driven by de novo downregulation of pro-inflammatory signaling networks, or handling could increase expression of anti-inflammatory signaling molecules, which in turn suppress transcription of pro-inflammatory mediators. To disambiguate these possibilities, we first measured TLR4 expression in the NAc of equivalently aged rats (from Figure 1) 14 days following cessation of self-administration, to investigate persistent changes in gene regulation. TLR4 is a critical initiator of pro-inflammatory responses, and there is considerable evidence that opioid compounds induce pro-inflammatory responses via TLR interactions, including TLR4 (Bachtell et al, 2015; Eidson et al, 2017; Wang et al, 2012). We found a significant main effect of drug treatment on TLR4 mRNA (F1,32=4.26, p<0.05) but no effect of early-life experience (Figure 3d); thus, remifentanil increases NAc expression of TLR4 regardless of early-life conditions. We next compared gene expression of anti-inflammatory IL-10, which broadly inhibits the expression of pro-inflammatory mediators released downstream of TLR ligation (Kishore et al, 1999; Saraiva and O'Garra, 2010). Consistent with previous work (Schwarz et al, 2011), we found IL-10 mRNA was elevated in the NAc of handled rats (Figure 3e), even after repeated remifentanil exposure (p<0.05; Kruskal–Wallis and Dunn’s test). Collectively, these results converge to support the notion that the early-life maternal environment can influence drug-induced cytokine/chemokine gene expression patterns in the NAc, which is characterized in part by increased anti-inflammatory gene expression.
Plasmid IL-10 Gene Therapy Increases IL-10 and Modifies Downstream Signaling
We next tested the hypothesis that genetic manipulation of anti-inflammatory IL-10 in the NAc is sufficient to attenuate opioid reinforcement in normal control rats. Naked pDNA encoding rat IL-10 (Figure 4a) has previously been delivered to the spinal cord to augment IL-10 transcription and effectively suppress glial pro-inflammatory cytokine production (Ledeboer et al, 2007), and delivery of D-mannose as a transgene adjuvant along with pDNA-IL-10 greatly improves plasmid transfection from a single injection (Dengler et al, 2014). To confirm the efficacy of pDNA-IL-10 treatment, we first measured IL-10 gene expression in vitro with dissociated rat brain cell cultures. Incubation with pDNA-IL-10+D-mannose increased IL-10 mRNA (F3,8=42.13, p<0.001), which was not observed with D-mannose alone or pDNA-control+D-mannose (Figure 4b). Furthermore, when microglia (CD11b+ cells) were isolated and cultured separately from CD11b− cells, pDNA-IL-10+D-mannose treatment increased IL-10 mRNA to a greater extent in the CD11b+ population (F3,15=4.01, p<0.05; Figure 4c). This suggests that, under in vitro conditions, microglia primarily (although not exclusively) internalize the pDNA vector and increase IL-10 transcription.
Naked pDNA encoding rat IL-10 delivered with D-mannose increases IL-10 expression in vitro and in vivo. (a) Design of plasmid vector (adapted from Milligan et al, 2006). (b) Using dissociated cell culture, incubation with D-mannose+pDNA-IL-10 for 2 h markedly increases IL-10 mRNA expression compared with D-mannose+pDNA-control or D-mannose alone (***p<0.001, Tukey’s post hoc test; n=3/group). (c). Isolated microglia (CD11b+ cells) show a greater increase in IL-10 mRNA following 2 h incubation with D-mannose+pDNA-IL-10, compared with the CD11b− population (*p<0.05 main effect of pDNA-IL-10 treatment; **p<0.01 compared with CD11b− population, Bonferroni’s post hoc test; n=2–3/group). (d) Left panel: Representative image of pDNA infusion site in NAc. Images acquired at × 10 magnification (Zeiss, Axio Imager) and tiling achieved using Zen 2 software (Zeiss); DAPI in blue, scale bar=1 mm. White arrowhead indicates infusion site, observed near the anterior commissure. Right panel: Representation of the stereotaxic coordinates for pDNA treatment into NAc based on the Paxinos and Watson atlas. Red line indicates path of Hamilton syringe. (e) IL-10 mRNA is elevated in striatal tissue 1 week following pDNA-IL-10 treatment (*p<0.05; n=5/group). (f) ERK1/2 activation (ratio of phosphorylated ERK1/2 to total ERK1/2 protein) is decreased in the striatum 1 week following pDNA-IL-10 treatment (***p<0.001; n=4/group).
To manipulate IL-10 in vivo, we stereotaxically delivered pDNA (IL-10 or control) with D-mannose bilaterally into the NAc (Figure 4d). One week later, NAc and adjacent striatal tissue was isolated and processed for mRNA and protein analyses. Treatment with pDNA-IL-10 significantly increased IL-10 mRNA compared with controls (t8=2.43, p<0.05; Figure 4e). We additionally precipitated protein from the majority of the same tissue samples and measured the phosphorylation state of extracellular signal-related kinase (ERK), a member of the mitogen-activated protein kinase (MAPK) family that is well characterized both for its involvement in the induction of IL-10 following pattern recognition receptor ligation on macrophages and dendritic cells (Saraiva and O'Garra, 2010), as well as being downstream of IL-10 signaling itself in the brain (Pereira et al, 2015). The ratio of phosphorylated ERK1/2 to total ERK1/2 was significantly decreased in vivo 1 week after rats received a single bilateral injection of pDNA-IL-10 into the NAc, compared with pDNA-control (t6=8.13, p<0.001; Figure 4f). These data demonstrate that the method of pDNA-IL-10 and D-mannose delivery utilized here, when administered in vitro and in vivo, effectively and persistently alters the transcriptional activity of IL-10 and functionally influences the phosphorylation state of a critical kinase for IL-10 signaling.
Plasmid IL-10 Delivery in the NAc Attenuates Remifentanil Self-Administration
To address the behavioral outcome of IL-10 gene therapy, we stereotaxically delivered pDNA (IL-10 or control) with D-mannose bilaterally into the NAc and measured self-administration for remifentanil at two drug concentrations (0.29 and 0.9 μg/kg) for 7 days each (Figure 5a). Remifentanil infusions acquired per session were measured over time (Figure 5b). A two-way RM-ANOVA comparing the first and final sessions for each drug concentration revealed a main effect of time on remifentanil consumption at 0.29 μg/kg (F1,16=6.90, p<0.05), which we suspect was related to increased food seeking on day 1. Importantly, there was no effect of pDNA treatment at this dose (Figure 5c). In contrast, at 0.9 μg/kg there was a main effect of time (F1,16=28.03, p<0.001) and a significant interaction between test day and pDNA treatment (F1,16=11.06, p<0.01). Post hoc comparisons revealed pDNA-IL-10 treatment significantly reduced remifentanil consumption on day 7 compared with pDNA-control treatment (p<0.05; Figure 5c). Responding on the inactive lever did not differ across sessions or between groups (Figure 5d and e).
Naked pDNA encoding rat IL-10 delivered into NAc reduces remifentanil self-administration. (a) Schematic representation of the remifentanil self-administration experimental timeline. Adult rats received bilateral stereotaxic delivery of D-mannose along with pDNA-control or pDNA-IL-10, and remifentanil self-administration behavior was observed (n=9/group). (b) The number of intravenous infusions of remifentanil was recorded in daily 1 h sessions (FR1, 20 s time-out) for 14 days: 7 days at 0.29 μg/kg and 7 days at 0.9 μg/kg. (c) pDNA-IL-10 treatment does not influence remifentanil self-administration during initial sessions at 0.29 μg/kg, although infusions per session did decrease from day 1 vs day 7. In contrast, pDNA-IL-10 significantly attenuated the number of remifentanil infusions acquired on day 7 when remifentanil is delivered at 0.9 μg/kg, compared with pDNA control (*p<0.05). (d) Responses on the inactive lever were recorded across sessions. (e) Inactive lever responding during remifentanil sessions did not differ between groups.
Self-Administration of Natural Rewards is not Altered by Neonatal Handling or Plasmid IL-10 Delivery in the NAc
To determine if the effect of neonatal handling on remifentanil administration is due to a generalized decrease in reward valuation, we assessed self-administration behavior for food or sucrose pellets in separate groups of handled and control rats from the same litters used for remifentanil (Supplementary Figure S2a). The number of pellets acquired per session rose dramatically over time both for food (Supplementary Figure S2b) and sucrose (Supplementary Figure S2e). There was a significant main effect of time for rats acquiring food (F1,13=69.22, p<0.001) and sucrose (F1,9=12.29, p<0.01). However, there was no effect of early-life experience on food (Supplementary Figure S2c) or sucrose (Supplementary Figure S2f) self-administration, and no differences between groups in their responding on the inactive lever during trials for food (Supplementary Figure S2d) or sucrose (Supplementary Figure S2g).
Similarly, food and sucrose self-administration was investigated following pDNA treatment (Supplementary Figure S3a). Responding for both food and sucrose was robust across sessions (Supplementary Figure S3b and e). The number of food pellets acquired per session increased over time (F1,8=9.87, p<0.05), but there was no effect of pDNA treatment on food intake (Supplementary Figure S3c). The number of sucrose pellets acquired did not change over time (presumably due to high initial responding as a result of previous food sessions), and was not affected by pDNA treatment (Supplementary Figure S3f). In addition, responding on the inactive lever did not differ across sessions or between groups for either food (Supplementary Figure S3d) or sucrose (Supplementary Figure S3g). These data suggest that neither handling nor pDNA-IL-10-induced attenuation of opioid self-administration generalize to food or sucrose.
Discussion
Our results reveal that the early-life maternal environment can influence later-life opioid self-administration in a drug-concentration-dependent manner. Moreover, we demonstrate that neonatal handling induces an anti-inflammatory transcriptional environment in the NAc after repeated remifentanil exposure and specifically increases anti-inflammatory IL-10 expression. We thus hypothesized that IL-10 expression may be a novel resilience factor for addiction liability. In support of this, direct administration of pDNA overexpressing IL-10 into the NAc of rats reduced self-administration of remifentanil in a dose- and time-dependent manner, without affecting the response to natural rewards. Importantly, these data are the first to our knowledge to demonstrate that gene therapy for an anti-inflammatory cytokine targeting a brain region critical for reward processing can influence opioid drug-taking behaviors.
Neonatal Handling and Opioid Reinforcement
Early-life experience (eg, stress or neglect) is one of the strongest predictors of substance abuse later in life (Dube et al, 2003; Felitti et al, 1998), though little is known about the long-lasting neurobiological mechanisms that shape an individual’s vulnerability or resilience for drug addiction. We demonstrate here that a reasonably simple manipulation of neonatal handling from P2 to P20 has a subtle yet significant effect on motivational behaviors for the fast-acting opioid remifentanil, attenuating the number of infusions acquired in a drug-concentration-dependent manner and resulting in a downward deflection of the dose–response curve. The enduring effects of neonatal handling on rodent physiological and behavioral sequelae have been known for decades (Levine, 1967), and many authors have proposed that variations in maternal care during this critical period of postnatal development (both naturally occurring variations and those induced by the experimenter through home-cage disruption) can influence neural circuit function throughout the lifespan (Curley and Champagne, 2016; Korosi and Baram, 2010; Meaney, 2001). Previous investigations have found neonatal handling to reduce cocaine and ethanol consumption (Moffett et al, 2006; Ploj et al, 2003), but its effect on opioid drug-taking was unknown. Here we report that neonatal handling attenuates remifentanil self-administration under defined conditions. Interestingly, the effect of handling was typically revealed when the remifentanil concentration was altered from the dose received in early sessions, implying a strong dose-dependent relationship (further discussion below). To this end, handling appears to shift the dose–response curve for remifentanil, where lower concentrations of drug are less reinforcing in the handled group. It is unlikely this response is due to a difference in tolerance, as the infusion rate at these lower doses did not fluctuate across sessions (Supplementary Figure S4); however, formal measures of drug tolerance were not assessed and cannot be entirely ruled out.
Neuroinflammatory Signaling and Opioid Reinforcement
The neuronal circuits projecting to and from the dopamine receptor-expressing neurons in the NAc have been extensively characterized for their role in motivation and reward learning (Hyman et al, 2006). Drug-induced synaptic plasticity in NAc medium spiny neurons is thought to contribute to the persistence of addiction behavior (Enoksson et al, 2012; Graziane et al, 2016; Hearing et al, 2016; Pascoli et al, 2014), and μ-opioid receptor-expressing neurons in the NAc are also critical for the maintenance of remifentanil consumption (Cui et al, 2014). More recently, microglial cytokine signaling in the NAc has been associated with synaptic and behavioral responses to morphine (Schwarz and Bilbo, 2013) as well as cocaine (Lewitus et al, 2016). On the basis of these collective observations, we focused our characterization of neuroimmune gene expression to the NAc; however, it remains to be determined if the transcriptional results observed here are specific to the NAc. Distinct glial and neuroimmune contributions to morphine tolerance and withdrawal have been identified in the ventral tegmental area (Taylor et al, 2016), periaqueductal gray (Eidson et al, 2017), and spinal cord (Burma et al, 2017), suggesting that neuroimmune adaptations occur across distributed neural circuits and contribute to diverse outcomes associated with repeated opioid exposure.
Our transcriptional analyses suggest that cytokine and chemokine signaling within the NAc, including pathways associated with TLRs, may represent important neural-glial communication associated with opioid reinforcement. For instance, we found that remifentanil self-administration increases TLR4 expression in the NAc regardless of early-life experience. Opioids can interact with the lipopolysaccharide-binding pocket of myeloid differentiation factor-2 (MD-2), leading to TLR4 oligomerization and subsequent pro-inflammatory cytokine and chemokine transcription (Wang et al, 2012). Non-specific modulators of inflammatory signaling (eg, ibudilast) as well as specific disruption of TLR4 signaling reduces the rewarding effects of opioids, potentially through reduced NAc dopamine transmission (Bland et al, 2009; Hutchinson et al, 2012). The contribution of TLR signaling to addiction-like behavior is supported by studies in which disruption of TLR4 signaling prevents morphine and oxycodone conditioned place preference and reduces remifentanil self-administration (Hutchinson et al, 2012). Similarly, chronic antagonism of TLR4 with (+)-naltrexone reduces cue-induced heroin self-administration following withdrawal (Theberge et al, 2013). Given that TLR4/MD-2 signaling may also underlie behavioral responding to cocaine (Northcutt et al, 2015), TLR activity could represent a unifying mechanism regulating the immune responses to drugs of abuse, and inhibition of the pro-inflammatory downstream products may alter drug reinforcement.
Despite the promising view of TLR4 as a master regulator of opioid-induced neuroinflammation, some conflicting reports have emerged regarding its function in opioid-induced behavioral effects, including morphine withdrawal and remifentanil self-administration (Mattioli et al, 2014; Tanda et al, 2016). In light of these observations, alternative immune signaling pathways may also be recruited to mediate drug-induced inflammation. TLR2, for instance, has also been implicated in mediating microglial inflammatory responses to opioids (Zhang et al, 2011), cocaine (Liao et al, 2016), and ethanol (Fernandez-Lizarbe et al, 2013), and we observed TLR2 gene downregulation specifically in handled rats following remifentanil. There were additional unexpected findings from the transcriptional profiling, such as significant elevations of IL-6 in drug-naive handled rats. Given the pro- and anti-inflammatory properties of IL-6 in classic- and trans-signaling (Scheller et al, 2011), it is difficult to predict the functional outcomes this would support. Although the precise mechanisms of opioid-induced neuroinflammation remain unclear, further research aimed at cell-type-specific perturbations of neuroimmune signaling may help resolve these issues.
The Role of IL-10 in Opioid Reinforcement
We confirm that neonatal handling produces a persistent elevation in IL-10 gene expression in the NAc, consistent with previous reports (Schwarz et al, 2011). IL-10 is a potent inhibitor of TLR-mediated signaling and can directly inhibit cytokine transcription and translation (Kishore et al, 1999). The selectivity to which IL-10 can inhibit TLR4 is further demonstrated by the characterization of microRNA-146b, which is induced by IL-10 and negatively regulates TLR4-mediated pro-inflammatory cytokine and chemokine induction (Curtale et al, 2013). Thus, we predicted that elevated expression of IL-10 may be sufficient to suppress the downstream signaling pathways of TLR activation in response to opioids, either directly inhibiting TLR activity or indirectly influencing gene expression patterns of other critical cytokines and chemokines, including those identified in the PCR array. The data derived from the functional pathway analysis supports this assumption, as functionally related inflammatory genes from the PCR array show a strong, consistent pattern of downregulation in the NAc from handled rats.
On the basis of the data presented here, we predicted that augmentation of IL-10 expression in non-handled rats would recapitulate the behavioral results induced by neonatal handling. Because we sought to determine if IL-10 upregulation itself is sufficient to influence behavior, regardless of early-life experience, we chose to treat only non-handled subjects. We administered naked pDNA-IL-10 due to its proven efficacy for gene therapy while avoiding some immunogenicity concerns from viral transfection (Milligan et al, 2006; Yin et al, 2014). Although transfection efficiency of pDNA is a major challenge, D-mannose as a transgene adjuvant greatly improves transgene expression, potentially by stimulating phagocytosis (Dengler et al, 2014). Indeed, we provide evidence that pDNA-IL-10 treatment with D-mannose increases IL-10 mRNA both in vitro and in vivo. IL-10 mRNA was upregulated in vivo 1 week following treatment and was accompanied by changes in ERK protein phosphorylation, suggestive of persistent changes at the levels of cytokine transcription and downstream effectors (Pereira et al, 2015). Whether these pDNA-induced changes in IL-10 and ERK phosphorylation persist throughout self-administration awaits further exploration.
Following confirmation of our pDNA-IL-10 treatment paradigm, we present novel evidence that direct gene manipulation of an anti-inflammatory cytokine can influence opioid reinforcement. Interestingly, this response was drug-concentration-dependent in a manner similar to the neonatal handling condition. We predict that microglia may play a critical role in these outcomes, given that acute morphine administration profoundly increases IL-10 transcription primarily in microglial cells of the NAc (Schwarz et al, 2013). Previous analysis of isolated NAc microglia determined that neonatal handling results in epigenetic alterations to the DNA methylation state of IL-10 (Schwarz et al, 2011), which may explain the remarkably persistent effects of maternal care on IL-10 expression into adulthood. Likewise, we found in vitro treatment with pDNA-IL-10 significantly increases IL-10 mRNA expression to a greater extent in the CD11b+ microglial population. This suggests that cellular internalization of the pDNA vector occurs primarily in microglia. However, additional experiments are required to determine the cellular localization of the IL-10 transgene in vivo. Although it is currently unclear if other immunocompetent cells of the central nervous system (such as astrocytes or meningeal T cells) are influenced by or contribute to the behavioral consequences observed following neonatal handling or pDNA manipulation, our results are consistent with the interpretation that anti-inflammatory signaling in the brain can influence behavioral responses to opioids.
Limitations and Conclusions
There are limitations inherent in experimental design that are worth considering. Although the handling conditions were identical preceding remifentanil self-administration (Figures 1 and 2), there are important differences in remifentanil exposure between the experiments, including number of sessions, doses received, and the sequence of its availability. There is considerable evidence that the extent of drug exposure (in terms of dose and frequency of exposure) can impact subsequent behavioral and molecular responses to the drug (Ahmed et al, 2000; Mendrek et al, 1998; Robinson and Kolb, 2004). Thus, we cannot rule out the possibility that differences in the quantity and duration of remifentanil exposure could explain some of the behavioral or transcriptional effects observed. For instance, handled rats on the dose–response curve show attenuated drug intake during sessions at lower concentrations (0.09 and 0.29 μg/kg), whereas the significant effect of handling was revealed only when the dose was increased from 0.29 to 0.9 μg/kg. This suggests that the concentration of intravenous remifentanil received per infusion during initial sessions shapes the outcome of responding to subsequent changes in drug concentration, and this outcome can be influenced by early-life environmental conditions. Indeed, there is evidence that drug dose and session time during training or early sessions can influence drug consumption in subsequent trials for abused substances, including cocaine and heroin (Mandt et al, 2012; Martin et al, 1998). Although these differences in experimental design prevent direct comparisons between results, the data converge on a hypothesis of anti-inflammatory signaling in the NAc as a characteristic of early-life experience that influences opioid reinforcement, which was elaborated upon by pDNA-IL-10 experiments. Further delineation of how early-life experience or IL-10 gene therapy influences opioid intake over time in a drug-concentration-dependent manner is a topic of considerable interest that warrants further characterization.
An additional caveat to note is that differences in cytokine and chemokine gene expression in Handled-Remi rats (Figure 3) could be a consequence of decreased drug exposure, as opposed to handling per se. Future experiments employing yoked, non-contingent delivery of remifentanil could offer additional insight into the molecular consequences that occur when drug intake is controlled. Moreover, attenuation of remifentanil drug-taking could potentially be explained by altered motivation to positive reinforcers, or a behavioral deficit to operant conditioning. However, this notion was not supported by the data, as neither handling nor pDNA-IL-10 treatment influenced food or sucrose self-administration. Although the experimental timeline differed from rats receiving remifentanil (ie, no jugular catheterization surgery), the results suggest that handling and intracranial pDNA-IL-10 treatment do not suppress motivation for palatable rewards.
In conclusion, utilizing both environmental and genetic manipulations aimed at altering IL-10 expression, we identify an anti-inflammatory mechanism that is associated, under certain conditions, with resilience against the reinforcing properties of opioids. These data support the expanding literature implicating neuroimmune signaling from glia and other sources as important modulators of behavioral outcomes associated with drug addiction (Lacagnina et al, 2017). Future research aimed at modifying immune responses with increased precision might offer additional insights to improve therapeutics for those already suffering from opioid abuse.
Funding and disclosure
This work was supported by the National Institute of Health Grant DA034185 (SBD), the National Health and Medical Research Council CJ Martin Fellowship ID 1054091 (PMG), and the American Australian Association Sir Keith Murdoch Fellowship (PMG). MJL, AMK, SSC, RH, CW, SS, PMG, EDL and SDB declare no competing financial interest. LRW is a co-founder of Xalud Therapeutics and a co-chair of the Xalud Therapeutics Scientific Advisory Committee.
References
Ahmed SH, Walker JR, Koob GF (2000). Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22: 413–421.
Bachtell R, Hutchinson MR, Wang X, Rice KC, Maier SF, Watkins LR (2015). Targeting the toll of drug abuse: the translational potential of Toll-like receptor 4. CNS Neurol Disord Drug Targets 14: 692–699.
Bilbo SD, Newsum NJ, Sprunger DB, Watkins LR, Rudy JW, Maier SF (2007). Differential effects of neonatal handling on early life infection-induced alterations in cognition in adulthood. Brain Behav Immun 21: 332–342.
Bland ST, Hutchinson MR, Maier SF, Watkins LR, Johnson KW (2009). The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain Behav Immun 23: 492–497.
Burma NE, Bonin RP, Leduc-Pessah H, Baimel C, Cairncross ZF, Mousseau M et al (2017). Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat Med 23: 355–360.
Calcaterra S, Glanz J, Binswanger IA (2013). National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999-2009. Drug Alcohol Depend 131: 263–270.
Cui Y, Ostlund SB, James AS, Park CS, Ge W, Roberts KW et al (2014). Targeted expression of μ-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nat Neurosci 17: 254–261.
Curley JP, Champagne FA (2016). Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Front Neuroendocrinol 40: 52–66.
Curtale G, Mirolo M, Renzi TA, Rossato M, Bazzoni F, Locati M (2013). Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci USA 110: 11499–11504.
Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56.
Dengler EC, Alberti LA, Bowman BN, Kerwin AA, Wilkerson JL, Moezzi DR et al (2014). Improvement of spinal non-viral IL-10 gene delivery by D-mannose as a transgene adjuvant to control chronic neuropathic pain. J Neuroinflammation 11: 92.
Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF (2003). Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiencesstudy. Pediatrics 111: 564–572.
Eidson LN, Inoue K, Young LJ, Tansey MG, Murphy AZ (2017). Toll-like receptor 4 mediates morphine-induced neuroinflammation and tolerance via soluble tumor necrosis factor signaling. Neuropsychopharmacology 42: 661–670.
Enoksson T, Bertran-Gonzalez J, Christie MJ (2012). Nucleus accumbens D2- and D1-receptor expressing medium spiny neurons are selectively activated by morphine withdrawal and acute morphine, respectively. Neuropharmacology 62: 2463–2471.
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V et al (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14: 245–258.
Fernandez-Lizarbe S, Montesinos J, Guerri C (2013). Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J Neurochem 126: 261–273.
Fernando ABP, Robbins TW (2011). Animal models of neuropsychiatric disorders. Annu Rev Clin Psychol 7: 39–61.
Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK (2016). New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol 17: 34–40.
Graziane NM, Sun S, Wright WJ, Jang D, Liu Z, Huang YH et al (2016). Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci 19: 915–925.
Hall BJ, Slade S, Allenby C, Kutlu MG, Levin ED (2015). Neuro-anatomic mapping of dopamine D1 receptor involvement in nicotine self-administration in rats. Neuropharmacology 99: 689–695.
Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA et al (2016). Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci USA 113: 757–762.
Huang DW, Sherman BT, Lempicki RA (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57.
Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J et al (2012). Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 32: 11187–11200.
Hyman SE, Malenka RC, Nestler EJ (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29: 565–598.
Kishore R, Tebo JM, Kolosov M, Hamilton TA (1999). Cutting edge: clustered AU-rich elements are the target of IL-10-mediated mRNA destabilization in mouse macrophages. J Immunol 162: 2457–2461.
Kopec AM, Rivera PD, Lacagnina MJ, Hanamsagar R, Bilbo SD (2017). Optimized solubilization of TRIzol-precipitated protein permits Western blotting analysis to maximize data available from brain tissue. J Neurosci Methods 280: 64–76.
Korosi A, Baram TZ (2010). Plasticity of the stress response early in life: mechanisms and significance. Dev Psychobiol 52: 661–670.
Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR (2015). The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology 96: 55–69.
Lacagnina MJ, Rivera PD, Bilbo SD (2017). Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharmacology 42: 156–177.
Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED et al (2007). Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun 21: 686–698.
Levin ED, Hall BJ, Chattopadhyay A, Slade S, Wells C, Rezvani AH et al (2016). Reduction of nicotine self-administration by chronic nicotine infusion with H1 histamine blockade in female rats. Psychopharmacology 233: 3009–3015.
Levine S (1967). Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science 156: 258–260.
Lewitus GM, Konefal SC, Greenhalgh AD, Pribiag H, Augereau K, Stellwagen D (2016). Microglial TNF-α suppresses cocaine-induced plasticity and behavioral sensitization. Neuron 90: 483–491.
Liao K, Guo M, Niu F, Yang L, Callen SE, Buch S (2016). Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J Neuroinflammation 13: 33.
Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A et al (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277: 1659–1662.
Lüscher C, Malenka RC (2011). Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69: 650–663.
Mandt BH, Gomez E, Johnston NL, Zahniser NR, Allen RM (2012). Cocaine dose and self-administration history, but not initial cocaine locomotor responsiveness, affects sensitization to the motivational effects of cocaine in rats. J Pharmacol Exp Ther 342: 214–221.
Martin TJ, Smith JE, Dworkin SI (1998). Training dose and session time as contextual determinants of heroin self-administration in rats. Pharmacol Biochem Behav 60: 415–421.
Mattioli TA, Leduc-Pessah H, Skelhorne-Gross G, Nicol CJ, Milne B, Trang T et al (2014). Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PLoS ONE 9: e97361.
Meaney MJ (2001). Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24: 1161–1192.
Mendrek A, Blaha CD, Phillips AG (1998). Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology 135: 416–422.
Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J (2008). Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry 13: 208–221.
Miguel-Hidalgo JJ (2009). The role of glial cells in drug abuse. Curr Drug Abuse Rev 2: 72–82.
Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG et al (2006). Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain 126: 294–308.
Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI, Kuhar MJ (2006). Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J Pharmacol Exp Ther 317: 1210–1218.
Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A (2001). Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765.
Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA et al (2015). DAT isn't all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry 20: 1525–1537.
Panlilio LV, Schindler CW (2000). Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology 150: 61–66.
Pascoli V, Terrier J, Espallergues J, Valjent E, O'Connor EC, Lüscher C (2014). Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509: 459–464.
Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates 6th edn. Academic Press/Elsevier: Amsterdam/Boston.
Pereira L, Font-Nieves M, Van den Haute C, Baekelandt V, Planas AM, Pozas E (2015). IL-10 regulates adult neurogenesis by modulating ERK and STAT3 activity. Front Cell Neurosci 9: 57.
Ploj K, Roman E, Nylander I (2003). Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience 121: 787–799.
Ransohoff RM (2016). A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19: 987–991.
Robinson TE, Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291.
Robinson TE, Kolb B (2004). Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47 (Suppl 1): 33–46.
Rudd RA, Aleshire N, Zibbell JE, Gladden RM (2016). Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep 64: 1378–1382.
Saraiva M, O'Garra A (2010). The regulation of IL-10 production by immune cells. Nat Rev Immunol 10: 170–181.
Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S (2011). The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813: 878–888.
Schwarz JM, Bilbo SD (2013). Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. J Neurosci 33: 961–971.
Schwarz JM, Hutchinson MR, Bilbo SD (2011). Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci 31: 17835–17847.
Schwarz JM, Smith SH, Bilbo SD (2013). FACS analysis of neuronal-glial interactions in the nucleus accumbens following morphine administration. Psychopharmacology 230: 525–535.
Tanda G, Mereu M, Hiranita T, Quarterman JC, Coggiano M, Katz JL (2016). Lack of specific involvement of (+)-naloxone and (+)-naltrexone on the reinforcing and neurochemical effects of cocaine and opioids. Neuropsychopharmacology 41: 2772–2781.
Taylor AM, Castonguay A, Ghogha A, Vayssiere P, Pradhan AA, Xue L et al (2016). Neuroimmune regulation of GABAergic neurons within the ventral tegmental area during withdrawal from chronic morphine. Neuropsychopharmacology 41: 949–959.
Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM et al (2013). Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry 73: 729–737.
Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K et al (2012). Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci USA 109: 6325–6330.
Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD (2011). Microglia and memory: modulation by early-life infection. J Neurosci 31: 15511–15521.
Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG (2014). Non-viral vectors for gene-based therapy. Nat Rev Genet 15: 541–555.
Zhang Y, Li H, Li Y, Sun X, Zhu M, Hanley G et al (2011). Essential role of toll-like receptor 2 in morphine-induced microglia activation in mice. Neurosci Lett 489: 43–47.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Lacagnina, M., Kopec, A., Cox, S. et al. Opioid Self-Administration is Attenuated by Early-Life Experience and Gene Therapy for Anti-Inflammatory IL-10 in the Nucleus Accumbens of Male Rats. Neuropsychopharmacol 42, 2128–2140 (2017). https://doi.org/10.1038/npp.2017.82
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2017.82
This article is cited by
-
Microglia in neuroimmunopharmacology and drug addiction
Molecular Psychiatry (2024)
-
Neuroinflammatory Response in Reward-Associated Psychostimulants and Opioids: A Review
Cellular and Molecular Neurobiology (2023)
-
Transgenerational Susceptibility to Food Addiction-Like Behavior in Rats Associates to a Decrease of the Anti-Inflammatory IL-10 in Plasma
Neurochemical Research (2022)
-
Amitifadine, a triple reuptake inhibitor, reduces self-administration of the opiate remifentanil in rats
Psychopharmacology (2020)
-
Inflammation Predicts Decision-Making Characterized by Impulsivity, Present Focus, and an Inability to Delay Gratification
Scientific Reports (2019)