Abstract
Alterations in cortical parvalbumin (PV)-containing neurons, including a reduced density of detectable neurons and lower PV levels, have frequently been reported in the dorsolateral prefrontal cortex (DLPFC) of schizophrenia subjects. Most PV neurons are surrounded by perineuronal nets (PNNs) and the density of PNNs, as detected by Wisteria floribunda agglutinin (WFA) labeling, has been reported to be lower in schizophrenia. However, the nature of these PNN alterations, and their relationship to disease-related changes in PV neurons, has not been assessed. Using confocal microscopy, we quantified the densities and fluorescence intensities of PV neurons and PNNs labeled with WFA or immunoreactive for the major PNN protein, aggrecan, in the DLPFC from schizophrenia and matched comparison subjects. In schizophrenia, the densities of PV cells and of PNNs were not altered; however, the fluorescence intensities of PV immunoreactivity in cell bodies and of WFA labeling and aggrecan immunoreactivity in individual PNNs around PV cells were lower. These findings indicate that the normal complements of PV cells and PNNs are preserved in schizophrenia, but the levels of PV protein and of individual PNN components, especially the carbohydrate moieties on proteoglycans to which WFA binds, are lower. Given the roles of PV neurons in regulating DLPFC microcircuits and of PNNs in regulating PV cellular physiology, the identified alterations in PV neurons and their PNNs could contribute to DLPFC dysfunction in schizophrenia.
Similar content being viewed by others
INTRODUCTION
Among the many clinical features of schizophrenia, deficits in cognitive functions, including working memory, appear to reflect the core disease process (Kahn and Keefe, 2013). Working memory requires the synchronized activity of specific microcircuits in layer 3 of the dorsolateral prefrontal cortex (DLFPC) (Arnsten, 2013), consisting of reciprocal connections between excitatory pyramidal cells (PCs) and a subset of GABAergic interneurons that express the calcium-binding protein parvalbumin (PV) (Curley and Lewis, 2012). Consistent with working memory impairments in schizophrenia, multiple post-mortem studies have demonstrated morphological and/or molecular alterations in DLPFC layer 3 PCs and PV neurons in schizophrenia subjects. These alterations include reduced dendritic spine density on PCs, specifically in deep layer 3 (Glantz and Lewis, 2000), lower levels of PV mRNA specifically in layers 3 and 4 (Fung et al, 2010; Hashimoto et al, 2003), and reduced PV protein in PV basket cell terminals in layer 3 (Glausier et al, 2014). Lower levels of PV mRNA and protein could be explained by a loss of PV neurons, which are highly enriched in cortical layers 3 and 4 (Hashimoto et al, 2003; Tooney and Chahl, 2004), and/or reduced levels of PV in some cells. However, studies investigating PV cell density in schizophrenia have provided mixed results (Beasley et al, 2002; Hashimoto et al, 2003; Tooney and Chahl, 2004; Woo et al, 1997).
Most cortical PV neurons, and a small number of PCs, are ensheathed by perineuronal nets (PNNs), a condensed form of extracellular matrix (Berretta et al, 2015) involved in the closure of developmental critical periods, regulation of synaptic plasticity, and are altered by oxidative stress (Cabungcal et al, 2013b; McRae and Porter, 2012). Several PNN proteoglycans, the lecticans, are highly glycosylated, and one, aggrecan, is highly enriched in mature PNNs (Bitanihirwe and Woo, 2014). The density of PNNs labeled with Wisteria floribunda agglutinin (WFA), a lectin that binds to specific carbohydrate moieties on proteoglycans, has been reported to be markedly lower in the entorhinal and prefrontal cortices in schizophrenia (Mauney et al, 2013; Pantazopoulos et al, 2010, 2015).
A lower density of PNNs in schizophrenia could be the consequence of one or more different pathological processes. First, a lower density of PNNs could be the result of fewer PV neurons for PNNs to surround. Second, fewer PNNs could reflect a failure in PNN formation and/or maintenance, which would be seen as a loss of multiple PNN markers and a lower proportion of PV neurons surrounded by PNNs, without a reduction in PV cell density. Third, the detectability of PNNs could be reduced because of alterations in their composition (eg, lower glycosylation of aggrecan).
To differentiate among these possibilities, we used quantitative, high-resolution imaging and an unbiased stereological sampling approach. PNNs were labeled with WFA and an antibody that recognizes aggrecan, in combination with markers of PV neurons and PCs, in DLPFC layer 3 from schizophrenia and healthy comparison subjects as layer 3 has the highest density of both PV neurons (Hashimoto et al, 2003) and PNNs (Mauney et al, 2013). The density of PV-positive cells and the intensity of PV labeling per neuron were quantified along with the proportion of PV cells surrounded by PNNs. In addition the relative levels of aggrecan and WFA labeling in individual PNNs were determined.
MATERIALS AND METHODS
Human Subjects
All tissue specimens (n=56) were donated to the University of Pittsburgh Brain Tissue Donation Program following consent of next of kin as described previously (Glausier et al, 2015). Each subject with schizophrenia (n=20) or schizoaffective disorder (n=8) was matched to one unaffected comparison subject for sex and as closely as possible for age and post-mortem interval (PMI). Subject groups did not differ in mean age, PMI, or tissue storage time (Table 1 and Supplementary Table 1). See Supplementary Methods for details.
Tissue Processing and Immunohistochemistry
The left hemisphere of each brain was blocked coronally at ~12 mm thickness, immersed for 48 h in cold 4% paraformaldehyde, cryoprotected, and stored at −30 °C. Blocks containing DLPFC area 9 were sectioned along the rostral–caudal axis at 40 μm. Tissue sections at a similar rostral–caudal level were randomly selected from each member of a subject pair, and both sections were processed together. Two immunohistochemical runs containing 13 and 15 subject pairs, respectively, were conducted. In both immunohistochemical runs, fluorescence immunohistochemistry was performed using the lectin WFA and antibodies against PV and aggrecan, followed by fluorescent Nissl staining. In immunohistochemical run 2, a NeuN antibody was also used to enhance identification of PCs. See Supplementary Methods for details.
Antipsychotic-Treated Monkeys
Four male long-tailed macaque monkeys were treated chronically for 9–12 months with haloperidol decanoate as described previously (Pierri et al, 1999). Each animal was matched to an untreated comparison animal based on sex, age, and weight. Tissue sections from the mid-portion of the principal sulcus were processed for immunohistochemistry as described above, except that the aggrecan and NeuN antibodies were not used. See Supplementary Methods for details.
Stereological Methods and Image Capture
Before low magnification imaging, a power analysis of the number of cells needed to detect a difference in PV cell density, based on prior findings (Beasley et al, 2002), was used to determine the total number of PV neurons to sample. All slides were coded so that the experimenter was blinded to diagnosis. For low magnification imaging, a × 20 objective (0.75 NA) was used; high magnification imaging was performed using a super-corrected × 60 oil objective (1.4 NA). For the monkey study, sampling and imaging at high and low magnification were performed in a manner similar to the human study. See Supplementary Methods for details.
Image Processing
Imaging and processing were performed similar to the previous studies (Fish et al, 2011; Rocco et al, 2015). Specifically, exposure times during image capture were optimized to prevent saturating conditions for each channel in each image stack and differences in exposure times were corrected before processing. Images were subjected to deconvolution and intensity histograms for each channel were independently adjusted to identical settings across all subjects allowing PV-positive cells and PNNs to be masked under identical conditions across all subjects. These approaches have been previously used to obtain quantitative measures of immunofluorescence intensity across a wide dynamic range (Curley et al, 2011; Fish et al, 2011; Glausier et al, 2015). See Supplementary Methods for details.
Statistical Analyses
Two analyses of covariance (ANCOVA) models (paired and unpaired) were used as described previously (Glausier et al, 2015) to test the effect of diagnosis on each dependent variable. All reported values are from the paired ANCOVA as both models yielded similar results (except where noted in the text).The results from all ANCOVA models are presented in Supplementary Table 2. See Supplementary Methods for details (including assessment of comorbid factors).
RESULTS
PV and PNN Labeling is Prominent in DLPFC Layer 3
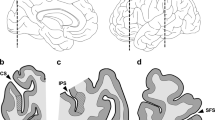
In the comparison subjects, qualitative analyses revealed that PV neurons and PNNs (identified by WFA labeling or aggrecan immunoreactivity) were present in greatest density in layer 3 of DLPFC area 9 (Figure 1a-1), consistent with prior findings (Hashimoto et al, 2003; Mauney et al, 2013). Many PV neurons were surrounded by PNNs and the majority of PNNs surrounded PV neurons; however, some PCs were also surrounded by PNNs (Figure 1b-4). High magnification analyses confirmed that WFA and aggrecan labeling colocalized within nearly all PNNs (Figure 1c–4).
Perineuronal nets (PNNs) are present around parvalbumin (PV) and pyramidal neurons in human dorsolateral prefrontal cortex (DLPFC). Images of NeuN (1—left column), PV (2—middle left column), aggrecan (3—middle right column) and Wisteria floribunda agglutinin (WFA) labeling (4—right column) in DLPFC area 9. (a) Representative images (using a x 4 objective) showing the high density of PV neurons and aggrecan- and WFA-labeled PNNs in layer 3. Numbers indicate the locations of the cortical layers. Scale bar=300 μm. (b) Low magnification images (using a × 20 objective) in layer 3 showing pyramidal cells with PNNs (arrowheads), PV neurons with PNNs (arrows), and PV neurons lacking PNNs (notched arrowheads). Scale bar=30 μm. (c) High magnification images (using a × 60 objective) showing that most PV neurons are ensheathed by PNNs (arrows) detected with both aggrecan and WFA labeling and that some PV neurons (notched arrowheads) lack PNNs labeled with either aggrecan or WFA. Scale bar=10 μm. WM, white matter.
Apparent Lower PV Cell Density in Schizophrenia Reflects a Reduced Intensity of PV Immunolabeling
Under low magnification, the mean (±SD) density of layer 3 PV cells in schizophrenia subjects (2695±527 cells per mm3) was 11.7% lower (F1,27=7.54, P=0.011; Figure 2a) compared with that in comparison subjects (3051±416 cells per mm3), a decrement similar to a previous study (Beasley et al, 2002). However, under high magnification, mean PV cell density did not differ (F1,20=0.0, P=0.88; Figure 2b) between schizophrenia (3032±634 cells per mm3) and comparison (3060±605 cells per mm3) subjects. The difference in the PV cell density findings between low and high magnification studies could be attributed to lower PV labeling intensity per neuron. PV cell density did not differ between the two magnifications in comparison subjects, whereas in schizophrenia subjects lower PV expression may render some neurons undetectable at low magnification. Consistent with this interpretation, under high magnification the mean fluorescence intensity of PV immunoreactivity in PV cell bodies surrounded by aggrecan-positive PNNs was 25.6% lower (F1,27=10.0, P=0.004; Figure 2c) in schizophrenia (424.5±183.7 arbitrary units (a.u.)) compared with that in comparison (570.9±269.4 a.u.) subjects. In the PV cells lacking aggrecan PNNs, mean soma PV levels were also 25% lower (F1,27=6.0, P=0.021; Figure 2d) in schizophrenia subjects (501.9±235.6) compared with that in comparison subjects (668.8±339.3).
Parvalbumin (PV) density is unchanged, but PV levels are lower in schizophrenia subjects. (a–d) Unity plots in which each data point represents a subject pair and data points below the unity line indicate lower values in the schizophrenia subject relative to the matched comparison subject. (a) The density of PV cells seen under low magnification is lower in schizophrenia subjects. (b) The density of PV cells seen under high magnification is not affected by disease status. Soma PV labeling in PV cells in schizophrenia subjects is lower in (c) PV cells with an aggrecan perineuronal nets (PNNs) and (d) PV cells lacking a PNN.
Interestingly, PV cells lacking PNNs were smaller than those with PNNs in both subject groups (Figure 3a and b, t55=27.6, P<0.0001, paired T-test for all subjects) and there was no effect of diagnosis on PV soma size in cells with (F1,27=2.2, P=0.147; Figure 3c) or lacking PNNs (F1,27=2.2, P=0.148; Figure 3d). Furthermore, mean soma PV levels were positively correlated between cells surrounded by or lacking PNNs within both comparison (r=0.667, P<0.01) and schizophrenia (r=0.666, P<0.01) subjects, suggesting that PV levels in schizophrenia are lower in PV cells, regardless of the presence of a PNN. These data indicate that PV cell density is not altered in schizophrenia, but that lower PV protein levels make PV cells more difficult to detect under low magnification.
Parvalbumin (PV) cells with perineuronal nets (PNNs) are larger than PV cells lacking PNNs. (a) Representative dual-color images at high magnification of individual PV (red) cells with aggrecan (green) PNNs (top two rows) and lacking PNNs (bottom two rows) from different healthy comparison subjects. Scale bar=10 μm. (b) Distribution of soma cross-sectional area (cells were placed into 40 μm2 bins) from healthy subjects of all PV cells with (dark gray) and without (light gray) aggrecan PNNs showing that PV cells surrounded by PNNs are larger than PV cells lacking a PNN. (c) Distribution of soma cross-sectional area from all PV cells with PNNs (cells were placed into 40 μm2 bins) from healthy (black) and schizophrenia (dark gray) subjects. (d) Distribution of soma cross-sectional area from all PV cells (cells were placed into 20 μm2 bins) without PNNs from healthy (black) and schizophrenia (dark gray) subjects. In (c and d) there was no effect of diagnosis on soma cross-sectional area.
Labeling Intensity, but not Density, of PNNs is Lower in Schizophrenia
Because these data suggest that reports of lower PNN density in the DLPFC of schizophrenia subjects (Mauney et al, 2013) is not due to a loss of PV cells, we investigated whether the proportion of PV cells with PNNs was altered in schizophrenia. At low magnification, the mean percentage of PV-positive cells with WFA-positive PNNs in schizophrenia subjects (53.5±24.7%) was lower (F1,27=6.1, P=0.02) compared with that in comparison subjects (64.6±20.4%), although this did not achieve statistical significance in the unpaired ANCOVA (Supplementary Table 2). The mean percentage of PV-positive cells with aggrecan-positive PNNs (Figure 4b) was also lower (F1,26=13.0, P=0.001) in schizophrenia (76.7±5.6%) relative to comparison subjects (80.6±3.8%), although the magnitude of the difference was much smaller than the reduction seen for WFA-positive PNNs. The much higher percentage of PV cells with aggrecan-positive PNNs compared with WFA-positive PNNs in both subject groups suggested that the apparent lower density of PNNs in schizophrenia under low magnification could reflect reduced PNN labeling and not a loss of PNN structure.
Parvalbumin (PV) cell perineuronal net (PNN) density is unchanged, but PNN labeling is lower in schizophrenia subjects. Unity plot showing under low magnification that the proportion of PV cells with a Wisteria floribunda agglutinin (WFA) (a) or aggrecan (b) PNN is lower in schizophrenia subjects. (c) At high magnification the proportion of all PV cells with an aggrecan PNN is not affected by disease status. The average aggrecan (d) and WFA (e) fluorescence intensity from aggrecan PNNs around PV cells is lower in schizophrenia subjects. The closed data point in (e) represents the subject pair identified as an extreme outlier that was removed from this and subsequent high magnification WFA analyses. (f) There is a strong correlation between the % PV cells with a WFA PNN seen under low magnification and the intensity of WFA PNN fluorescence under high magnification.
To investigate this possibility, we quantified the proportion of PV cells with aggrecan-positive PNNs and the fluorescence intensity of aggrecan and WFA labeling within PNNs at high magnification. The proportion of PV neurons with aggrecan-labeled PNNs did not differ (F1,27=0.556, P=0.463) between comparison (78.6±6.4%) and schizophrenia (77.4±7.6%) subjects (Figure 4c) and nearly all aggrecan-positive PNNs around PV cells were also labeled with WFA, regardless of diagnosis. In contrast, the mean fluorescence intensity of aggrecan immunoreactivity (Figure 4d) was 30.8% lower (F1,27=15.9; P<0.001) in schizophrenia (221.7±83.1 a.u.) relative to comparison (320.1±131.2 a.u.) subjects. Similarly, the mean fluorescence intensity of WFA labeling in PNNs (Figure 4e) was 48.0% lower (F1,26=8.2, P=0.008) in schizophrenia (158.7±166.0 a.u.) compared with that in comparison (305.4±295.0 a.u.) subjects (Supplementary Table 2). Taken together, these findings indicate that PNNs surrounding PV neurons are preserved in schizophrenia, but that lower levels of PNN components render them more difficult to detect under low magnification. Consistent with this interpretation, the intensity of WFA labeling in PNNs at high magnification and the proportion of PV cells surrounded by WFA-labeled PNNs at low magnification were positively correlated across all subjects (r=0.41, P=0.002; Figure 4f), demonstrating that lower fluorescence intensity of PNN labeling with WFA leads to the appearance of fewer WFA-labeled PNNs at low magnification.
The Effects of Comorbid Factors on PV and PNN Labeling
After Bonferroni correction for multiple comparisons (n=7 dependent measures, resulting in a corrected significant P-value=0.0071) within each comorbid factor, no measures of PV or PNN labeling differed between schizophrenia subjects as a function of sex, tobacco use at the time of death, use of antidepressants or benzodiazepines and/or sodium valproate at the time of death, or a diagnosis of schizoaffective disorder (Supplementary Table 3). Schizophrenia subjects who committed suicide had higher PV immunoreactivity in the soma of PV cells lacking PNNs (Supplementary Table 3), a finding of uncertain significance. Furthermore, we saw no significant correlation between any dependent variable and age or post-mortem interval in either diagnostic group (P>0.05 by Pearson’s correlation). In addition, no dependent measures differed within subject pairs between schizophrenia subjects who were on or off antipsychotics at the time of death. Because only four schizophrenia subjects were off antipsychotics at the time of death, we looked at potential effects of these medications in adult monkeys chronically exposed to the antipsychotic haloperidol. Haloperidol treatment was not associated with alterations in the proportion of PV cells with WFA PNNs (control 87.8±5.4%, haloperidol treated 88.8±2.4%, P=0.748), the intensity of WFA labeling in individual PNNs (control 271.8±55.0, haloperidol treated 236.7±14.1, P=0.573), or somal PV labeling of PV cells with PNNs (control 911.1±73.7, haloperidol treated 920.4±113.4, P=0.958). In concert, these data suggest that the findings of reduced PNN and PV labeling in schizophrenia subjects is unlikely due to comorbid factors.
DISCUSSION
PV Cells and PNNs are Normal in Density but More Difficult to Detect in Schizophrenia Subjects
The mixed reports of lower cortical PV cell density in schizophrenia could be due to reduced PV expression, rendering some PV cells more difficult to detect under the conditions of some studies (Stan and Lewis, 2012). Indeed, under low magnification, we observed a deficit (11.7%) in the density of PV cells similar to a previous report (Beasley et al, 2002). However, under high magnification low-intensity objects should be easier to detect as the × 60 objective used in this study has a 25% higher brightness index and a nearly 50% increase in resolving power compared with the × 20 objective. Indeed, under high magnification PV cell density did not differ between subject groups, whereas the intensity of PV immunoreactivity per neuron was lower in schizophrenia, a pattern concordant with findings at the mRNA level (Hashimoto et al, 2003). Furthermore, the low and high magnification analyses detected nearly identical densities of PV cells in comparison subjects (compare Figures 2a and 3b), indicating that cells with normal levels of PV protein are readily detectable. Thus, prior reports of apparently lower PV cell density (Beasley et al, 2002) are likely due to lower levels of PV protein and reported losses of PNNs (Mauney et al, 2013) are not likely due to loss of PV neurons.
Similarly, the lower proportion of PV cells with PNNs observed under low magnification in schizophrenia subjects likely reflects the reduced intensity of PNN labeling seen under high magnification, without a loss of PNNs. First, at low magnification the deficit in the proportion of PV cells with aggrecan PNNs was much smaller than with WFA labeling, suggesting that aggrecan labeling is relatively less affected in schizophrenia subjects. Second, because PNN-positive PV cells have larger somata than those lacking PNNs, a loss of PNNs (without a loss of PV neurons) in schizophrenia would result in a greater mean somal size of PNN-negative PV cells; however, such a difference between subject groups was not observed. Last, WFA labeling intensity of individual PNNs seen under high magnification was strongly positively correlated with the percentage of PV cells surrounded by a WFA PNN seen at low magnification. Taken together, these findings are consistent with both the persistence of PV cells and PNNs in schizophrenia and a greater sensitivity of aggrecan immunoreactivity than WFA labeling for detecting PNNs. Although the density of PV cells and of the PNNs that surround them are not lower in schizophrenia, lower levels of PV, aggrecan, and WFA labeling clearly indicate a pathological process in this component of DLPFC layer 3 circuitry.
The lower levels of PV protein and of PNN markers appear to reflect the disease process and not factors that are frequently comorbid with schizophrenia such as suicide, tobacco use, and use of psychotropic medications. As only four schizophrenia subjects in this study were off antipsychotic medications at the time of death and at least two had taken these medications in the past, we examined the potential effects of antipsychotics on PV and PNNs in monkeys chronically exposed to the antipsychotic haloperidol. In these animals, we found no effect of antipsychotic treatment on WFA labeling or PV immunoreactivity. Although this approach does not permit the detection of a disease by drug interaction, these data suggest that antipsychotic medications alone cannot account for the observed deficits in PV and WFA labeling in schizophrenia subjects.
What Mechanisms Might Account for Both Lower PV Levels and Lower PNN Labeling in Schizophrenia?
Levels of PV (Kinney et al, 2006) and PNN labeling (Dityatev et al, 2007; Giamanco and Matthews, 2012) are regulated in an activity-dependent manner. For example, increased PV and WFA labeling during the closure of critical periods in sensory cortices is likely driven by increased excitatory input (McRae and Porter, 2012). In addition, both aggrecan expression (McRae et al, 2010) and glycosylation state (Lander et al, 1997) are activity-dependent. Because DLPFC layer 3 PCs appear to receive fewer excitatory synaptic inputs (Glantz and Lewis, 2000) resulting in a lower expression of gene products critical for energy production (Arion et al, 2015), their lower activity would result in less excitatory drive to neighboring layer 3 PV cells (which receive a large proportion of their excitatory inputs from layer 3 PCs (Melchitzky et al, 1998). A reduction in excitatory inputs could result in an activity-dependent downregulation of PV and PNN components in schizophrenia. Consistent with this interpretation, neuronal activity in sensory cortices during the closure of a critical period is associated with both the maturation of PV neurons and the appearance of PNNs (Nowicka et al, 2009; Ye and Miao, 2013).
Alternatively, other mechanisms might alter PNNs, which in turn could lead to altered PV levels in schizophrenia. For example, altered sulfation of N-acetylgalactosamine (GalNAc, a major disaccharide found in CSPGs) results in greatly reduced WFA labeling (Miyata et al, 2012). Furthermore, PNNs might be subject to enhanced degradation in schizophrenia. Interestingly, the expression of several enzymes involved in extracellular matrix (ECM) degradation is increased in the temporal cortex in schizophrenia (Pietersen et al, 2014). Moreover, two distinct populations of PV neurons have recently been interrogated based on the presence of PNNs, and the PV cells with PNNs (which are the majority of PV neurons) are highly enriched for markers involved in matrix remodeling (Rossier et al, 2015), suggesting these cells may be particularly susceptible to ECM alterations. Any of these mechanisms could lead to lower PV levels as experimental disruptions of PNNs results in lower PV mRNA expression (Yamada et al, 2015), without a change in the number of detectable PV cells (Cabungcal et al, 2013a), findings that match the current report. In contrast, our findings of a positive correlation between PV and PNN labeling is unlikely to be due to lower PV expression causing lower PNN labeling because mice lacking PV have normal WFA labeling (Schwaller et al, 2004).
Functional Consequences of Lower Levels of PV and PNN Components
Our observation that the level of PV protein per neuron, and not the number of PV neurons, is lower in schizophrenia may have the following functional consequences. PV is a low-affinity calcium buffer that likely regulates neurotransmitter release (Eggermann and Jonas, 2012). Furthermore, PV is important in short-term plasticity in striatal neurons and loss of PV can affect the excitability and output of these cells (Orduz et al, 2013). Moreover, mice lacking PV have behavioral deficits, including altered social interactions (Wohr et al, 2015). Therefore, reductions in PV could result in altered GABA release and inhibition of pyramidal neurons in the DLPFC and contribute to the clinical features of schizophrenia.
Alterations in PNNs in schizophrenia could also have substantial adverse effects on the function of the affected neurons. First, PNNs carry a high degree of negative charge and may function as cation sinks (Bruckner et al, 1993). This role may be particularly important for PV neurons to maintain their fast-spiking properties. Second, the ECM is critical for establishing the local Cl− concentration (Glykys et al, 2014). Therefore, PNN disruption could alter inhibitory inputs to layer 3 PV neurons. Third, the loss of ECM components increases the mobility of excitatory AMPA receptors (Frischknecht et al, 2009), potentially altering the ability of glutamatergic inputs to depolarize the cell. Fourth, disruption of PNNs can alter the resting membrane potential of PV neurons (Miyata et al, 2012) and decrease the amplitudes of both inhibitory (Liu et al, 2013) and excitatory (Pyka et al, 2011) postsynaptic currents. Each of these factors could affect the excitability of PV neurons and contribute to lower expression of activity-dependent genes, such as Zif268 and GAD67 (Kimoto et al, 2014), in PV neurons. Several studies suggest that ECM components, including PNNs, are critical regulators of synaptic plasticity (Do et al, 2015; Miyata et al, 2012; Morishita et al, 2015). Finally, PNNs appear to be both protective against and altered by high levels of oxidative stress (Cabungcal et al, 2013a; Do et al, 2015; Morishita et al, 2015). Higher levels of oxidative stress have been reported in schizophrenia (Wang et al, 2009; Yao et al, 2006) and increased oxidative stress in an animal model results in lower WFA labeling of PNNs and alterations in γ-oscillations (Cabungcal et al, 2013a, b).
Taken together, these data show that a normal complement of PV neurons and the PNNs that surround them are present in the DLPFC of subjects with schizophrenia. However, decreased expression of both PV and PNN components are present in schizophrenia, suggesting that PV cell dysfunction is part of the disease process. Through a variety of mechanisms, PV and PNN-mediated alterations are likely to contribute to dysfunction of the layer 3 PV-PC local circuitry that generates γ-oscillations (Gonzalez-Burgos et al, 2015) and thus to the neural basis for the impairments in DLPFC γ-oscillations seen during cognitive tasks in individuals with schizophrenia (Uhlhaas and Singer, 2015).
Funding and disclosure
David A Lewis currently receives investigator-initiated research support from Pfizer. In 2013–2015, he served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals, and Sunovion. The other authors declare no conflict of interest.
References
Arion D, Corradi JP, Tang S, Datta D, Boothe F, He A et al (2015). Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry 20: 1397–1405.
Arnsten AFT (2013). The neurobiology of thought: the groundbreaking discoveries of Patricia Goldman-Rakic 1937–2003. Cereb Cortex 23: 2269–2281.
Beasley CL, Zhang ZJ, Patten I, Reynolds GP (2002). Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry 52: 708–715.
Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET (2015). Losing the sugar coating: potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res 167: 18–27.
Bitanihirwe BK, Woo TU (2014). Perineuronal nets and schizophrenia: the importance of neuronal coatings. Neurosci Biobehav Rev 45: 85–99.
Bruckner G, Brauer K, Hartig W, Wolff JR, Rickmann MJ, Derouiche A et al (1993). Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8: 183–200.
Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ (2013a). Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry 73: 574–582.
Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK et al (2013b). Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci USA 110: 9130–9135.
Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN et al (2011). Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry 168: 921–929.
Curley AA, Lewis DA (2012). Cortical basket cell dysfunction in schizophrenia. J Physiol 590 (Part 4): 715–724.
Dityatev A, Bruckner G, Dityateva G, Grosche J, Kleene R, Schachner M (2007). Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol 67: 570–588.
Do KQ, Cuenod M, Hensch TK (2015). Targeting oxidative stress and aberrant critical period plasticity in the developmental trajectory to schizophrenia. Schizophr Bull 41: 835–846.
Eggermann E, Jonas P (2012). How the ‘slow’ Ca(2+) buffer parvalbumin affects transmitter release in nanodomain-coupling regimes. Nat Neurosci 15: 20–22.
Fish KN, Sweet RA, Lewis DA (2011). Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cereb Cortex 21: 2450–2460.
Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED (2009). Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci 12: 897–904.
Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS (2010). Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry 167: 1479–1488.
Giamanco KA, Matthews RT (2012). Deconstructing the perineuronal net: cellular contributions and molecular composition of the neuronal extracellular matrix. Neuroscience 218: 367–384.
Glantz LA, Lewis DA (2000). Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 57: 65–73.
Glausier JR, Fish KN, Lewis DA (2014). Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry 19: 30–36.
Glausier JR, Kimoto S, Fish KN, Lewis DA (2015). Lower glutamic acid decarboxylase 65-kDa isoform messenger RNA and protein levels in the prefrontal cortex in schizoaffective disorder but not schizophrenia. Biol Psychiatry 77: 167–176.
Glykys J, Dzhala V, Egawa K, Balena T, Saponjian Y, Kuchibhotla KV et al (2014). Local impermeant anions establish the neuronal chloride concentration. Science 343: 670–675.
Gonzalez-Burgos G, Cho RY, Lewis DA (2015). Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry 77: 1031–1040.
Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z et al (2003). Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 23: 6315–6326.
Kahn RS, Keefe RS (2013). Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry 70: 1107–1112.
Kimoto S, Bazmi HH, Lewis DA (2014). Lower expression of glutamic acid decarboxylase 67 in the prefrontal cortex in schizophrenia: contribution of altered regulation by Zif268. Am J Psychiatry 171: 969–978.
Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM (2006). A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci 26: 1604–1615.
Lander C, Kind P, Maleski M, Hockfield S (1997). A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex. J Neurosci 17: 1928–1939.
Liu H, Gao PF, Xu HW, Liu MM, Yu T, Yao JP et al (2013). Perineuronal nets increase inhibitory GABAergic currents during the critical period in rats. Int J Ophthalmol 6: 120–125.
Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S et al (2013). Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry 74: 427–435.
McRae PA, Baranov E, Sarode S, Brooks-Kayal AR, Porter BE (2010). Aggrecan expression, a component of the inhibitory interneuron perineuronal net, is altered following an early-life seizure. Neurobiol Dis 39: 439–448.
McRae PA, Porter BE (2012). The perineuronal net component of the extracellular matrix in plasticity and epilepsy. Neurochem Int 61: 963–972.
Melchitzky DS, Sesack SR, Pucak ML, Lewis DA (1998). Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J Comp Neurol 390: 211–224.
Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H (2012). Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci 15: 414–422 S411–S412.
Morishita H, Cabungcal JH, Chen Y, Do KQ, Hensch TK (2015). Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biol Psychiatry. 78: 396–402.
Nowicka D, Soulsby S, Skangiel-Kramska J, Glazewski S (2009). Parvalbumin-containing neurons, perineuronal nets and experience-dependent plasticity in murine barrel cortex. Eur J Neurosci 30: 2053–2063.
Orduz D, Bischop DP, Schwaller B, Schiffmann SN, Gall D (2013). Parvalbumin tunes spike-timing and efferent short-term plasticity in striatal fast spiking interneurons. J Physiology 591 (Part 13): 3215–3232.
Pantazopoulos H, Markota M, Jaquet F, Ghosh D, Wallin A, Santos A et al (2015). Aggrecan and chondroitin-6-sulfate abnormalities in schizophrenia and bipolar disorder: a postmortem study on the amygdala. Transl Psychiatry 5: e496.
Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S (2010). Extracellular matrix–glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry 67: 155–166.
Pierri JN, Chaudry AS, Woo TU, Lewis DA (1999). Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry 156: 1709–1719.
Pietersen CY, Mauney SA, Kim SS, Lim MP, Rooney RJ, Goldstein JM et al (2014). Molecular profiles of pyramidal neurons in the superior temporal cortex in schizophrenia. J Neurogenet 28: 53–69.
Pyka M, Wetzel C, Aguado A, Geissler M, Hatt H, Faissner A (2011). Chondroitin sulfate proteoglycans regulate astrocyte-dependent synaptogenesis and modulate synaptic activity in primary embryonic hippocampal neurons. Eur J Neurosci 33: 2187–2202.
Rocco BR, Lewis DA, Fish KN (2015). Markedly Lower Glutamic Acid Decarboxylase 67 Protein Levels in a Subset of Boutons in Schizophrenia. Biol Psychiatry (e-pub ahead of print; doi:10.1016/j.biopsych.2015.07.022).
Rossier J, Bernard A, Cabungcal JH, Perrenoud Q, Savoye A, Gallopin T et al (2015). Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol Psychiatry 20: 154–161.
Schwaller B, Tetko IV, Tandon P, Silveira DC, Vreugdenhil M, Henzi T et al (2004). Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol Cell Neurosci 25: 650–663.
Stan AD, Lewis DA (2012). Altered cortical GABA neurotransmission in schizophrenia: insights into novel therapeutic strategies. Curr Pharm Biotechnol 13: 1557–1562.
Tooney PA, Chahl LA (2004). Neurons expressing calcium-binding proteins in the prefrontal cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 28: 273–278.
Uhlhaas PJ, Singer W (2015). Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol Psychiatry 77: 1001–1009.
Wang JF, Shao L, Sun X, Young LT (2009). Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord 11: 523–529.
Wohr M, Orduz D, Gregory P, Moreno H, Khan U, Vorckel KJ et al (2015). Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Transl Psychiatry 5: e525.
Woo TU, Miller JL, Lewis DA (1997). Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry 154: 1013–1015.
Yamada J, Ohgomori T, Jinno S (2015). Perineuronal nets affect parvalbumin expression in GABAergic neurons of the mouse hippocampus. Eur J Neurosci 41: 368–378.
Yao JK, Leonard S, Reddy R (2006). Altered glutathione redox state in schizophrenia. Dis Markers 22: 83–93.
Ye Q, Miao QL (2013). Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol 32: 352–363.
Acknowledgements
We thank Mary Brady for her excellent technical assistance. This work was supported by National Institutes of Health Grants MH043784 (DAL) and MH096985 (KNF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Enwright, J., Sanapala, S., Foglio, A. et al. Reduced Labeling of Parvalbumin Neurons and Perineuronal Nets in the Dorsolateral Prefrontal Cortex of Subjects with Schizophrenia. Neuropsychopharmacol 41, 2206–2214 (2016). https://doi.org/10.1038/npp.2016.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2016.24
This article is cited by
-
Altered excitatory and inhibitory ionotropic receptor subunit expression in the cortical visuospatial working memory network in schizophrenia
Neuropsychopharmacology (2024)
-
Modulators of GABAA receptor-mediated inhibition in the treatment of neuropsychiatric disorders: past, present, and future
Neuropsychopharmacology (2024)
-
Perineuronal Nets Alterations Contribute to Stress-Induced Anxiety-Like Behavior
Molecular Neurobiology (2024)
-
Epidermal Growth Factor Suppresses the Development of GABAergic Neurons Via the Modulation of Perineuronal Net Formation in the Neocortex of Developing Rodent Brains
Neurochemical Research (2024)
-
Pharmacogenetic activation of parvalbumin interneurons in the prefrontal cortex rescues cognitive deficits induced by adolescent MK801 administration
Neuropsychopharmacology (2023)