Abstract
The mammalian basolateral amygdala (BLA) and medial prefrontal cortex (mPFC) comprise a functionally interconnected circuit that is critical for processing opiate-related associative memories. In the opiate-naïve state, reward memory formation in the BLA involves a functional link between dopamine (DA) D1 receptor (D1R) and extracellular signal-related kinase 1/2 (ERK1/2) signaling substrates, but switches to a DA D2 (D2R)/Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα)-dependent memory substrate following chronic opiate exposure and spontaneous withdrawal. Using conditioned place preference (CPP) in rats paired with molecular analyses, we examined the role of intra-mPFC CaMKII, ERK and DAergic activity during the formation of opiate associative memories, and how opiate exposure state may regulate the functions of these molecular memory pathways. We report that the role of CaMKIIα signaling is functionally reversed within the BLA-mPFC pathway depending on opiate exposure state. Thus, in the opiate-naïve state, intra-mPFC but not intra-BLA blockade of CaMKII signaling prevents formation of opiate reward memory. However, following chronic opiate exposure and spontaneous withdrawal, the role of CaMKII signaling in the BLA-mPFC is functionally reversed. This behavioral memory switch corresponds to a selective increase in the expression of D2R and CaMKIIα, but not other calcium/calmodulin-related molecules, nor D1R expression levels within the mPFC.
Similar content being viewed by others
Introduction
The mammalian basolateral amygdala (BLA) and medial prefrontal cortex (mPFC) form a functionally interconnected circuit that is critical for the formation of memories linked to the rewarding properties of opiates (Bishop et al, 2011; Sun and Laviolette, 2012; Sun et al, 2011). Intra-BLA processing of opiate-related memories is mediated via dopamine (DA) D1 receptor (D1R) and D2R signaling as a function of opiate exposure state. Specifically, D1R transmission is required for acute opiate memory formation in the previously drug-naïve state, whereas D2R signaling is necessary for opiate memory formation during states of chronic opiate exposure and spontaneous withdrawal (Lintas et al, 2011, 2012).
Extracellular signal-related kinase 1/2 (ERK1/2) and the Ca2+/calmodulin-dependent protein kinase type II α isoform (CaMKIIα) are linked to DAergic activity and the formation of drug-related memories (Lyons et al, 2013). ERK signaling is associated with opiate reward and spontaneous withdrawal phenomena in mesocorticolimbic regions (Morón et al, 2010; Valjent et al, 2004). Furthermore, chronic opiate exposure leads to alterations in ERK signaling (Bilecki et al, 2005; Lyons et al, 2013). Activity of CaMKII is involved in reinstatement of morphine-seeking behaviors (Liu et al, 2012a, 2012b), and modifications to CaMKII expression are linked to opiate exposure differentially between acute and chronic treatments (Andersen et al, 2012; Lu et al, 2000; Lyons et al, 2013). Although both molecules interact with D1R and D2R, ERK signaling is related more closely to D1R-mediated effects (Fricks-Gleason and Marshall, 2011; Lai et al, 2008), whereas CaMKII directly modulates D2R function (Liu et al, 2009). Opiate exposure not only alters levels of ERK1/2 and CaMKII in the BLA, but ERK1/2 signaling is required for acute opiate memory formation in rats with no history of opiate exposure. In contrast, CaMKII-mediated signaling is necessary for memory formation following chronic opiate exposure and spontaneous withdrawal (Lyons et al, 2013).
The mPFC is involved in processing opiate-related memories and in generating neuronal responses to drug cues during relapse and drug seeking (Bossert et al, 2012; Doherty et al, 2013; Lucas et al, 2012; Pelloux et al, 2013; Sun et al, 2011). The BLA-mPFC circuit operates as an integrative, temporally mediated pathway during opiate-related associative memory processing (Bishop et al, 2011; Gholizadeh et al, 2013; Sun and Laviolette, 2012; Sun et al, 2011) and thus represents a crucial target for understanding the processing of opiate reward memories (Cardinal et al, 2002). We hypothesized that opiate-related molecular adaptations within the BLA-mPFC circuit may underlie the transition between opiate-naïve vs chronically exposed states, during the formation of opiate reward memories.
Using a combination of behavioral pharmacological and molecular analyses, we investigated the role of intra-mPFC CaMKII, ERK1/2, and DAergic signaling during the formation of opiate reward memories as a function of opiate exposure state. Remarkably, we report that chronic opiate exposure and spontaneous withdrawal induces a functional switch in the signaling pathways involved in the formation of opiate associative memories within the mPFC that are functionally opposite to those observed in the BLA. Thus, chronic opiate exposure and spontaneous withdrawal serves as a dissociative boundary between distinct DAergic and CaMKII/ERK memory substrates within the BLA-mPFC circuit.
Materials and Methods
Surgical Procedures
All procedures were performed in accordance with the Canadian Council on Animal Care and approved by the Western University Council on Animal Care. Male Sprague-Dawley rats (350–400 g; Charles River) were anesthetized with ketamine/xylazine (80 mg and 6 mg/kg, respectively), given 1 mg/kg of meloxicam (non-steroidal anti-inflammatory analgesic) and placed into a stereotaxic device. Stainless steel guide cannulae (22 gauge; PlasticsOne) were bilaterally implanted into the brain regions of interest based on anatomical boundaries defined by Paxinos and Watson (2005) as follows: mPFC (15° angle), from bregma, anteroposterior (AP) −2.9, mediolateral (ML) −1.9, from the dural surface, dorso-ventral (DV) −3.0; BLA (no angle), from bregma, AP −2.6, ML +5.0, DV −7.2. Rats in the mPFC-BLA disconnection groups received a single unilateral PFC cannulation and contralateral BLA cannulation. The hemispheres for unilateral cannulations were counterbalanced within groups to control for laterality.
Drug Treatments
The selective CaMKII inhibitor autocamtide-2-related inhibitory peptide (AIP; Tocris Bioscience), the D2/D3 antagonist eticlopride (eticlopride hydrochloride; Tocris Bioscience), morphine (morphine hydrochloride, MacFarlane Smith), and heroin (diacetyl-morphine, MacFarlane Smith) were dissolved in physiological saline, pH adjusted to 7.4. The selective ERK1 inhibitor (PD334581; Tocris Bioscience) was dissolved in a solution of 50% dimethyl sulfoxide (DMSO) and 50% physiological saline, pH adjusted to 7.4, and prepared at room temperature. Microinjections into mPFC or BLA (0.5 μl of volume per infusion) were delivered via plastic tubing connected to a 1 μl Hamilton microsyringe over 1 min. Injectors were left in situ for an additional 1 min to ensure diffusion from the injector tip. For all groups, microinfusions were performed immediately before injections of morphine or saline (i.p.) and subsequent placement in the conditioning environment. The dose of morphine used for conditioned place preference (CPP; 5 mg/kg, i.p.) is a supra-reward threshold conditioning dose, and produces robust behavioral CPP (Bishop et al, 2011; Lyons et al, 2013; Sun et al, 2011).
Chronic Opiate Exposure and Spontaneous Withdrawal
For rats in the chronic opiate exposure/spontaneous withdrawal condition, opiate exposure and spontaneous withdrawal was induced as previously described (De Jaeger et al, 2013; Lintas et al, 2011; Lyons et al, 2013). This regimen produces aversive motivational effects (conditioned place aversion), which are qualitatively similar to those produced following a 3-week morphine administration regimen (Nader et al, 1994; Bechara et al, 1995; Laviolette and van der Kooy, 2004). Rats received daily subcutaneous injections of 0.5 mg/kg heroin in their home cage starting 7 days before behavioral conditioning, and the first conditioning session began 21 h following their last heroin injection. Maintenance doses of heroin were administered during the conditioning phase 2.5 h following the end of each conditioning session, for a total of 15 injections over the course of an experiment. Rats in the opiate-naïve condition were yoked to chronic exposure/withdrawn animals with saline injections corresponding to heroin injections.
Place Preference Conditioning
A fully counterbalanced, unbiased CPP paradigm was used as described previously (Lintas et al, 2011; Lyons et al, 2013). Following surgical recovery, rats were pre-conditioned for 20 min in a motivationally neutral gray box and randomly assigned to an experimental group. The 8-day conditioning procedure commenced the following day. One conditioning environment was black with a Plexiglas floor that is wiped down with 0.25 ml of 2% acetic acid immediately before placing the rat into it. The alternating environment was white with a wire mesh and woodchip floor, so the environments differed in color, floor texture, and odor. These environments elicit no baseline preference in rats, as reported previously (Laviolette and van der Kooy, 2003). Rats received an equal number of morphine-paired and saline-paired conditioning sessions, each 30 min in duration (ie, four morphine environment pairings and four saline environment pairings over the 8-day procedure). Testing occurred 3–5 days following the final conditioning session in a drug-free state. The test environment contains both conditioning environments separated by a narrow, neutral gray zone. At the start of the 10-min test session, rats are placed in the neutral zone, and time spent in each environment is recorded and scored separately for each rat.
Histology
Following the completion of behavioral experiments, rats were deeply anesthetized with euthanyl (sodium pentobarbital, 240 mg/kg, i.p.) and transcardially perfused with 0.9% physiological saline, followed by 10% formalin. Once extracted, brains were refrigerated at 4 °C in a 25% sucrose in formalin solution for a minimum of 48 h before sectioning at 40 μm. Brain slices were stained with Cresyl Violet for verification of BLA and mPFC cannulae placements. Subjects with placements outside the anatomical boundaries as defined by Paxinos and Watson (2005) were excluded from analysis.
Western Blot Procedure
Two groups of rats received 15 daily injections of physiological saline or heroin (0.5 mg/kg, s.c.), equivalent to those received during behavioral conditioning. Tissue was extracted at 21 h following the final injection, and western blotting procedures were performed as outlined in Lyons et al, 2013. Primary antibody dilutions were as follows: α-tubulin (1 : 120 000; Sigma-Aldrich), phosphorylated ERK1/2 [T202/Y204] (pERK1/2; 1 : 2000; Cell Signaling Technology), total ERK1/2 (tERK1/2; 1 : 5000, Cell Signaling Technology), phosphorylated CaMKIIα [T286] (pCaMKIIα; 1 : 10 000; Cell Signaling Technology), total CaMKIIα (tCaMKIIα; 1 : 5000; Cell Signaling Technology), total CaMKIIβ (1 : 100; Santa Cruz Biotechnology), Ca2+/calmodulin-dependent protein kinase type IV (CaMKIV; 1 : 50 000; Sigma-Aldrich), calcineurin A (CnA; 1 : 100 000; Sigma-Aldrich), D1R (1 : 100; Santa Cruz Biotechnology), and D2R (1 : 100; Santa Cruz Biotechnology). Secondary antibodies (Thermo Scientific) were all used at a dilution of 1 : 20 000. Blots were incubated in a TBS-T solution with either 5% non-fat dried milk (Carnation) or 2.5% Bovine Serum Albumin Fraction V (Calbiochem) as recommended by the manufacturer.
Data Analysis
CPP data were analyzed with two-way repeated-measures analysis of variance (ANOVA) with a between-subjects factor of drug treatment, and a within-subjects factor of conditioning environment. Post-hoc analyses were performed with Bonferroni corrections where appropriate. Densitometry values for western blots were obtained with Kodak digital analysis software and analyzed with two-tailed t-tests.
Results
Effects of Chronic Heroin Exposure and Spontaneous Withdrawal on Calcium-Related Signaling Molecules in the mPFC
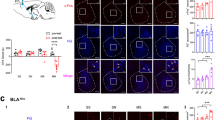
Given previous findings showing a profound reduction in intra-BLA CaMKIIα expression levels following chronic heroin exposure (Lyons et al, 2013), we first examined the protein expression profile of the CaMKIIα isoform in the mPFC as a function of opiate exposure state. Sample western blots presented in Figure 1a show representative phosphorylated and total CaMKIIα levels in opiate-naïve vs chronic exposure states. Rats chronically exposed to opiates and in spontaneous withdrawal at the time of tissue processing (n=8) showed no change in CaMKIIα phosphorylation levels compared with vehicle-treated rats (Figure 1b; n=8; t(14)=−0.446, p=0.662), but displayed significantly increased total CaMKIIα expression (t(14)=−3.152, p<0.01). As a result, the ratio of phosphorylated to total CaMKIIα was significantly decreased (Figure 1c; t(14)=3.850, p<0.01).
Chronic heroin exposure results in a state-dependent alteration in the expression of signaling molecules in the mPFC. (a) Representative western blot for phosphorylated and total CaMKIIα expression. (b) Densitometry analysis revealed no significant changes to phosphorylated CaMKIIα, but an increase in total CaMKIIα in opiate chronic exposure/withdrawn tissue. (c) The ratio of phosphorylated to total CaMKIIα is decreased in opiate chronic exposure/withdrawn animals relative to saline-treated animals. (d) Representative western blot for total CaMKIIβ. (e) Expression levels of CaMKIIβ are unchanged following chronic heroin exposure. (f) Representative western blot for total CaMKIV. (g) Levels of CaMKIV expression are not altered by chronic heroin exposure. (h) Representative western blot for CnA. (i) Expression levels of CnA are not changed by chronic heroin exposure (**p<0.01; error bars represent SEM).
In order to investigate potential changes to other isoforms of CaMKII, we next tested the CaMKIIβ isoform. A representative western blot is presented in Figure 1d. Analysis revealed no significant increase in total CaMKIIβ expression in chronic opiate exposure/withdrawn rats (n=8) compared with vehicle controls (n=8; t(14)=−1.383, p=0.188; Figure 1e). To determine if opiate exposure targets CaMKIIα specifically or Ca2+ (or Ca2+/calmodulin) more generally, we examined potential alterations to either CaMKIV (Figure 1f and g) or calcineurin A (CnA) (Figure 1h and i). There was no observed difference in the expression of CaMKIV or CnA between opiate-naïve vs chronically exposed/withdrawn rats (t(13)=0.153, p=0.881; t(12)=−0.075, p=0.941, respectively).
Histological Analysis for CPP Experiments
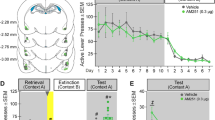
Histological analysis of intra-BLA and intra-mPFC cannula placements revealed microinjector tips within the anatomical boundaries of BLA and mPFC as outlined in Paxinos and Watson (2005). Figure 2 presents microphotographs and schematics of both intra-BLA (Figure 2a and b) and intra-mPFC (Figure 2c and d) placements of representative experimental groups. Histological analysis revealed intra-mPFC placements to be localized to the pre-limbic (PLC) subregion of the mPFC. Rats found to have placements outside the anatomical boundaries of the mPFC or BLA as defined by Paxinos and Watson (2005) were excluded from analyses.
Intra-mPFC and intra-BLA CaMKII signaling is functionally controlled by opiate exposure state. (a) Representative microphotograph of intra-BLA guide cannulae and microinjection tip placement. (b) Schematic of bilateral intra-BLA placements. Open triangles: 500 ng AIP in the opiate-naïve state; closed triangles 500 ng AIP in the chronic exposure/ spontaneous withdrawal state. (c) Representative microphotograph of intra-mPFC guide cannulae and microinjection tip placement. (d) Schematic of bilateral intra-mPFC placements. Open circles: 500 ng AIP in the opiate-naïve state; closed circles: 1 μg UO126 in the opiate chronic exposure/withdrawn state. (e) Intra-mPFC microinfusion of AIP dose-dependently blocked the acquisition of a morphine (0.5 mg/kg) CPP in a range from 5 ng to 500 ng in the opiate-naïve state. (f) Intra-mPFC microinfusion of 500 ng AIP did not block the acquisition of a morphine CPP in the opiate chronic exposure/withdrawn state. (g) In opposition to the mPFC, intra-BLA microinfusion of 500 ng AIP did not block the acquisition of a morphine CPP in rats trained in a previously opiate-naïve state; (h) however, intra-BLA microinfusion of 500 ng AIP blocked the acquisition of a morphine CPP in the opiate chronic exposure/withdrawn state (**p<0.01; error bars represent SEM).
Opiate Exposure State Controls the Functional Role of Intra-mPFC CaMKII Signaling during Opiate Reward Memory Formation
Given our observed changes in intra-mPFC CaMKIIα expression following chronic opiate exposure and spontaneous withdrawal, we next evaluated the behavioral significance of intra-mPFC CaMKII signaling across opiate exposure states. Here, we used a previously tested behaviorally effective dose range of the highly selective CaMKII inhibitor AIP (5–500 ng/0.5 μl) for bilateral intra-mPFC microinfusions (Lyons et al, 2013). First, using opiate-naïve experimental groups, intra-mPFC inhibition of CaMKII dose-dependently blocked the acquisition of morphine (5 mg/kg; i.p.) CPP (Figure 2e). Two-way repeated-measures ANOVA revealed a significant main effect of treatment (F(3, 25)=10.93, p<0.01), main effect of environment (F(1, 26)=14.55, p<0.01) and treatment by environment interaction (F(3, 22)=3.05, p<0.05). Post-hoc analyses revealed that whereas intra-mPFC vehicle (n=7) or the lowest dose of 5 ng AIP (n=6) were ineffective at blocking morphine CPP (p-values<0.01), both 50 ng (n=6) and 500 ng (n=7) doses blocked the formation of morphine CPP, with rats demonstrating no significant preference for morphine vs saline-paired environments (p-values>0.05).
We next examined the effects of intra-mPFC CaMKII inhibition on opiate reward behaviors in chronically exposed/withdrawn experimental groups (see Materials and methods section). Using the previously determined highest behaviorally effective dose of AIP (500 ng/0.5 μl; see Figure 2e), we found that intra-mPFC blockade of CaMKII failed to block morphine CPP. Although statistical analyses revealed a significant effect of group (F(1, 14)=7.21, p<0.05) and conditioning environment (F(1, 15)=68.44, p<0.01), both the groups receiving intra-mPFC AIP (n=7) and vehicle (n=8) demonstrated robust morphine CPP at testing (Figure 2f; p-values<0.01). In contrast to these findings in the mPFC, we have previously reported that intra-BLA CaMKII activity is not required for the acquisition of morphine CPP in the opiate-naïve state but, is necessary during states of chronic opiate exposure/spontaneous withdrawal (Lyons et al, 2013). Thus, to confirm the role of intra-BLA CaMKII signaling across opiate exposure states, we next examined the effects of intra-BLA CaMKII inhibition on opiate reward memory acquisition. Consistent with previous findings (Lyons et al, 2013), intra-BLA CaMKII inhibition in the opiate-naïve state failed to block morphine CPP relative to vehicle controls. Two-way repeated-measures ANOVA revealed a significant effect of conditioning environment (Figure 2g; F(1, 12)=86.59, p<0.01), and both the vehicle (n=7) and 500 ng AIP (n=5) groups showed significant place preference for the morphine-paired environment following behavioral conditioning (p-values<0.01). Inhibition of intra-BLA CaMKII, however, blocked the acquisition of morphine CPP in chronically exposed/withdrawn rats. Statistical analyses revealed a significant interaction of treatment group by environment between intra-BLA AIP and vehicle-treated groups (Figure 2h; F(1, 13)=6.441, p=0.02). Rats receiving 500 ng of AIP (n=8) showed no preference for morphine vs saline-paired environments (p>0.05) vs those receiving intra-BLA vehicle (n=7), who spent significantly more time in the morphine environment (p<0.01). Thus, within the BLA-mPFC circuit, blockade of CaMKII signaling reveals a behavioral double dissociation as a function of opiate exposure state: CaMKII signaling is required for intra-BLA opiate reward memory acquisition in the chronic opiate exposure/withdrawn state, but not naïve states. In contrast, intra-mPFC CaMKII signaling is required for opiate reward memory acquisition in the opiate-naïve, but not chronic exposure/withdrawn states.
Chronic Opiate Exposure and Spontaneous Withdrawal Alters the Expression and Function of Intra-mPFC D2R Receptor Transmission
Our present results show that intra-mPFC CaMKII signaling is required for opiate memory formation exclusively in the opiate-naïve state (Figure 2e) and that intra-mPFC CaMKIIα levels are markedly elevated in the opiate exposed and withdrawn state (Figure 1a and b). Remarkably, these results are in the opposite direction to those observed previously in the BLA wherein intra-BLA CaMKIIα signaling is functionally linked to D2R transmission only in the chronic opiate exposure/withdrawn state, concomitant with a significant downregulation in CaMKIIα levels (Lyons et al, 2013). Thus, we hypothesized that upregulation of CaMKIIα levels in the mPFC may be linked to opposing roles for intra-mPFC D2R transmission as a function of opiate exposure state. Accordingly, we next examined the effects of D2R blockade directly in the mPFC, comparing the opiate-naïve with chronic exposure/withdrawn states. Consistent with our hypothesis, intra-mPFC D2R blockade with the selective D2R antagonist, eticlopride (1 μg/0.5 μl; n=7) blocked acquisition of morphine CPP in the naïve, but not in the chronic exposure/withdrawn states (Figure 3a and b). For opiate-naïve groups, two-way repeated-measures ANOVA revealed a significant effect of conditioning environment (F(1, 14)=4.76, p<0.05), but no significant group effect (F(1, 13)=2.20, p=0.16; Figure 3a). However, post-hoc analyses revealed that rats treated with eticlopride (n=7) exhibited no preference for the morphine-paired environment (p>0.05), whereas vehicle controls (n=7) demonstrated significant CPP (p<0.05). In contrast, in chronically opiate exposed/withdrawn rats, intra-mPFC D2R inhibition failed to block morphine CPP. Two-way repeated-measures ANOVA revealed a significant main effect of conditioning environment (F(1, 12)=38.43, p<0.01; Figure 3b). Post-hoc analyses demonstrated that both vehicle-treated (n=7) and eticlopride-treated (n=5) groups exhibited significant preferences for morphine-paired environments (p-values<0.01, <0.05, respectively). Thus, blockade of D2R transmission in the mPFC selectively blocks morphine reward CPP in the opiate-naïve state, in direct contrast to the role of intra-BLA D2R transmission during opiate reward memory formation (Lyons et al, 2013). Given our observed behavioral dissociation in the role of intra-mPFC D2R transmission as a function of opiate exposure state, we next measured levels of intra-mPFC D2R protein expression between the two exposure states. A representative western blot of D2R expression levels is pictured in Figure 3c. Interestingly, levels of D2R expression showed a significant increase in chronically exposed/withdrawn rats (n=8) as compared with opiate-naïve (n=8) groups (Figure 3d; t(14)=−2.659, p=0.01), demonstrating that chronic opiate exposure/spontaneous withdrawal triggers a compensatory upregulation of D2R expression within the mPFC, concomitant with a lack of sensitivity to D2R blockade during the acquisition of opiate reward memories, relative to the opiate-naïve state.
Chronic heroin exposure alters function and expression of intra-mPFC D2R transmission. (a) Intra-mPFC microinfusion of 1 μg eticlopride blocked the acquisition of a morphine (5 mg/kg) CPP in the opiate-naïve state. (b) In the opiate chronic exposure/withdrawn state, a 1 μg eticlopride infusion into the mPFC did not block the acquisition of a 5 mg/kg morphine CPP. (c) Representative western blot of D2R expression in the mPFC between the opiate-naïve and chronic exposure/withdrawn states. (d) D2R expression is increased in the mPFC of opiate chronic exposure/withdrawn animals (*p<0.05, **p<0.01; error bars represent SEM).
Chronic Opiate Exposure Alters ERK1 Phosphorylation in the mPFC
Previous studies have demonstrated a state-dependent switch in the roles of the intra-BLA D1-ERK1/2 signaling pathway in the formation of opiate reward memories (Lintas et al, 2011; Lyons et al, 2013). Accordingly, we next tested for changes in protein expression and behavioral functions of the intra-mPFC D1-ERK1/2 pathway between the opiate-naïve and chronic exposure/withdrawn states. Representative western blots of phosphorylated and total ERK1/2 are presented in Figure 4a. Examining expression levels of intra-mPFC ERK isoforms across opiate exposure states revealed a significant increase in the expression of ERK1 phosphorylation between the opiate-naïve (n=7) and chronic exposure/withdrawn states (n=7; t(12)=−2.231, p<0.05; Figure 4b), but no change to total ERK1 levels (tERK1: t(12)=−0.650, p=0.361; Figure 4b). Consequently, the ratio of phosphorylated to total ERK1 expression was significantly increased in favor of pERK1 following chronic heroin exposure (t(12)=−2.646, p<0.05; Figure 4c). Levels of phosphorylated and total ERK2 in the chronic exposure/withdrawn state were not statistically different from the naïve state (Figure 4d; pERK2: t(12)=−1.232, p=0.241; tERK2: t(12)=0.110, p=0.914), and thus no change was seen in the ratio of phosphorylated to total ERK2 (Figure 4e; t(12)=−1.592, p=0.137). Given prior evidence demonstrating a functional relationship between alterations in intra-BLA ERK1/2 expression levels with the function of D1R transmission during opiate reward memory formation (Lyons et al, 2013), we next tested expression levels of the D1R directly within the mPFC. Western blots comparing the expression of DA D1Rs showed no difference in the expression of D1R between saline control and opiate chronic exposure/withdrawn rats (Figure 4f and g; t(12)=−0.317, p=0.756), demonstrating that, in contrast to the BLA, intra-mPFC alterations in ERK1 expression are not concomitant with changes in expression levels of the D1R.
Effect of chronic opiate exposure on the function and expression of ERK1/2 and expression of D1R in the mPFC. Panel (a) illustrates a representative western blot of phosphorylated ERK1/2 and total ERK 1/2. (b) Western blot analysis revealed a significant increase in phosphorylated ERK1, but not total ERK1 following chronic heroin exposure. (c) The ratio of phosphorylated to total ERK1 was significantly increased in opiate chronic exposure/withdrawn animals. (d) Levels of phosphorylated ERK2 and total ERK2 were not significantly altered in opiate chronic exposure/withdrawn groups. (e) The ratio of phosphorylated to total ERK2 was unchanged in opiate exposure/withdrawn animals. (f) Representative western blot of D1R expression in the mPFC. (g) Chronic heroin exposure does not alter the expression of D1R protein. Intra-mPFC microinfusion of 1 μg PD334581 did not block the acquisition of a morphine CPP in either the opiate-naïve state (h) or in the opiate chronic exposure/withdrawn state (i) (*p<0.05, **p<0.01; error bars represent SEM).
Intra-mPFC ERK1 Signaling is not Required for Opiate Memory Formation in Either the Opiate Naïve or the Opiate Chronic Exposure/Withdrawn States
To examine the potential behavioral significance of the observed alterations in pERK1 in chronic exposure/withdrawn mPFC tissue, we next tested the effect of ERK inhibition on the acquisition of a morphine CPP. Here, we used a pharmacologically selective ERK1 inhibitor, PD334581 (1 μg/0.5 μl), to directly examine the potential functional role of changes to pERK1 levels in opiate chronic exposure/withdrawn rats, given our findings that ERK1, but not ERK2 phosphorylation levels are altered as a function of chronic heroin exposure.
Two-way repeated-measures ANOVA revealed that selective inhibition of ERK1 in the mPFC had no effect on the acquisition of morphine CPP in the opiate-naïve state. There was no observed effect of treatment group (Figure 4h; F(1, 13)=2.08, p=v.17), but we did observe an effect of conditioning environment (F(1, 14)=39.45, p<0.01). Post-hoc analyses revealed that rats showed a significant morphine CPP following either intra-mPFC vehicle (n=7; p<0.01, as above) or PD334581 (n=7; p<0.01). Furthermore, inhibition of ERK1 in the mPFC had no effect on morphine CPP in the opiate chronic exposure/withdrawn state (Figure 4i). Statistical analyses revealed no effect of drug treatment (F(1, 13)=3.54, p=0.08) and a significant effect of conditioning environment (F(1, 14)=41.24, p<0.01). Groups receiving intra-mPFC microinfusion of vehicle (n=7) or 1 μg PD334581 (n=7) both demonstrated a significant preference for the morphine-paired environment (p-values<0.01).
Integrated ERK and CaMKII Signaling in the BLA-mPFC Circuit is Required for Opiate Reward Memory Acquisition
Thus far, the present results show that in the opiate-naïve state, intra-mPFC CaMKII (but not ERK) signaling is necessary for opiate reward memory formation. In contrast, in the BLA, opiate reward memory acquisition requires ERK (but not CaMKII) signaling specifically in the opiate-naïve state (Lyons et al, 2013). Given this BLA-mPFC dissociation and previous evidence suggesting that the processing of opiate reward memory requires functional connectivity between the BLA and mPFC (Gholizadeh et al, 2013; Sun and Laviolette, 2012), we next examined if integrated activity involving intra-BLA ERK and intra-mPFC CaMKII signaling is necessary for the acquisition of morphine reward memories. We tested this hypothesis by functionally disconnecting the effects of intra-BLA ERK signaling from intra-mPFC CaMKII signaling by simultaneously blocking intra-BLA ERK signaling in one hemisphere (U0126; 1 μg/0.5 μl) and intra-mPFC CaMKII signaling (AIP; 0.5 μg/0.5 μl) in the contralateral hemisphere (n=5) before morphine CPP conditioning (see Materials and methods section). In contrast, control groups received either intra-BLA combined with intra-mPFC vehicle infusions (n=7), intra-BLA ERK inhibition (U0126, 1 μg/0.5 μl, n=7) paired with intra-mPFC vehicle infusions, or intra-mPFC CaMKII inhibition (AIP; μg/0.5 μl) paired with intra-BLA vehicle infusions (n=6). A schematic representation of these experimental conditions is presented in Figure 5a.
Functional dissociation of the BLA-mPFC pathway in morphine CPP acquisition. (a) Schematic representation of experimental protocol for blocking the signaling molecules necessary for acquisition of a morphine CPP in the opiate-naïve state. (b) Unilateral intra-BLA microinfusion of 1 μg UO126 is insufficient to block the acquisition of a morphine (0.5 mg/kg) CPP, whereas unilateral intra-BLA microinfusion of 1 μg UO126 and contralateral inta-mPFC microinfusion of 500 ng AIP blocks the acquisition of a morphine CPP (**p<0.01; error bars represent SEM).
Two-way repeated-measures ANOVA revealed a significant interaction between conditioning environment and treatment (F(3, 21)=5.61, p<0.01; Figure 5b). Post-hoc analyses revealed that intra-BLA/intra-PFC vehicle microinfusions, unilateral blockade of intra-BLA ERK activity, and unilateral inhibition of intra-mPFC CaMKII activity all left the acquisition of a morphine CPP intact (p-values<0.01). Unilateral intra-BLA ERK blockade paired with contralateral mPFC CaMKII inhibition completely blocked the formation of morphine CPP, with rats showing no preference for morphine vs saline-paired environments at testing (p>0.05). Thus, contralateral disconnection of intra-BLA ERK from intra-mPFC CaMKII signaling during morphine CPP acquisition is sufficient to prevent morphine CPP reward memory formation.
Discussion
The BLA-mPFC circuit is critical for the processing of opiate-related reward information (Lintas et al, 2011, 2012; Lyons et al, 2013; Sun and Laviolette, 2012; Sun et al, 2011). Importantly, bi-directional connections between the BLA and mPFC have been demonstrated to strongly modulate opiate-related reward memory and learning at the behavioral, neuronal, and temporal levels of analysis. For example, at the cortical level, the activity patterns of mPFC neuronal sub-populations are correlated with the encoding, recall, and extinction of opiate-related reward memory (Sun and Laviolette, 2012; Sun et al, 2011). Functional connections between the BLA and mPFC are required for the temporal transfer of associative opiate reward memories and inactivation of the BLA modulates the neuronal activity dynamics of mPFC and regulates the acquisition and extinction of these memories (Gholizadeh et al, 2013; Sun and Laviolette, 2012). Nevertheless, the molecular mechanisms involved in BLA-mPFC circuit dynamics during the acquisition of opiate reward memories are not well understood.
Previous reports have demonstrated that opiate exposure state functions as a dynamic functional boundary between the roles of both the D1R and D2R systems and downstream molecular memory signaling molecules, including the ERK1/2 and CaMKIIα pathways, specifically within the BLA (Lintas et al, 2011; Lyons et al, 2013). Consequently, in the opiate-naïve state, acquisition of opiate reward memory requires ERK and D1R-dependent substrates within the BLA. However, following chronic opiate exposure and spontaneous withdrawal, intra-BLA opiate reward memory formation switches to a D2R, CaMKII-dependent signaling substrate (Lyons et al, 2013). In this study, we report that the role of the mPFC in the acquisition of opiate reward memories follows the opposite molecular memory pattern as a function of exposure state; that is, D2R-CaMKII-dependent signaling is required only in the opiate-naïve state, but not in the chronic exposure/withdrawn state, and both D2R and CaMKIIα expression levels are strongly increased as a function of chronic opiate exposure and spontaneous withdrawal. In stark contrast, in the BLA, chronic opiate exposure/spontaneous withdrawal induces a marked downregulation of CaMKIIα and ERK1/2 expression patterns (Lyons et al, 2013). Interestingly, although inhibition of ERK signaling in the BLA was shown previously to block opiate reward memory formation specifically in the opiate-naïve state (Lyons et al, 2013), in this study, inhibition of intra-mPFC ERK signaling was not sufficient to impair opiate reward memory formation, regardless of exposure state. This was the case despite the observation that chronic opiate exposure and spontaneous withdrawal induced an upregulation specifically in the ERK1 isoform within mPFC tissue. It is important to note that the intra-mPFC modulation of ERK1 levels shown here was substantially lower in magnitude to changes in intra-BLA ERK1/2 levels observed previously (Lyons et al, 2013) and, similar to CaMKIIα levels, was in the opposite direction (increased vs decreased expression levels as a function of opiate exposure state). Although beyond the scope of this study, an alternative possibility is that other signaling pathways may be able to compensate for ERK1 activity in the presence of ERK1 antagonism.
Forebrain CaMKIIα is necessary for contextual learning, specifically at the time of memory acquisition (Achterberg et al, 2014), consistent with our findings that intra-mPFC CaMKII inhibition during reward conditioning results in the blockade of morphine CPP acquisition. Furthermore, we have reported previously that intra-mPFC CaMKII signaling is necessary for the consolidation of acute opiate reward memories (Gholizadeh et al, 2013). At the molecular level, the observed increase in mPFC CaMKII is consistent with similar studies of region-specific increases to CaMKII following chronic morphine exposure (Chen et al, 2008; Narita et al, 2004). One study investigating potential biomarkers of chronic opiate exposure also found increased levels of Ca2+ in the serum of active heroin users (Divsalar et al, 2014). Interestingly, we observed a highly specific alteration in the total levels of the CaMKIIα isoform within the mPFC following chronic opiate exposure and spontaneous withdrawal, which was not observed in either the CaMKIIβ or CaMKIV isoforms. Furthermore, no significant alterations were observed in mPFC expression levels of calcineurin A. The present results show alterations in the functional role of the D2-CaMKII pathway, with CaMKII and D2 inhibition preventing the formation of a morphine reward memory in the naïve, but not the chronic exposure/withdrawn opiate exposure states. Whether chronic opiate exposure and spontaneous withdrawal results in alterations to D2 directly to affect downstream CaMKII activity, or whether CaMKII levels influence the expression of D2 is unknown. It has, however, been suggested that alterations in D2 receptor trafficking reduces the expression of intra-mPFC CaMKII (Papaleo et al, 2012) and not vice versa.
CaMKIIα signaling is functionally linked to D2R function. For example, previous reports have found that CaMKIIα is bound to an intracellular domain of the D2R both in vitro and in vivo, within the striatum (Zhang et al, 2014). Modulation of CaMKII activity controls D4 receptor function within the mPFC during the acquisition of fear-related memories (Lauzon et al, 2012) and CaMKIIα has been reported to control activity-dependent function of striatal D3 receptors by increasing their phosphorylation, thereby controlling D3 receptor-mediated responses to the psychostimulant effects of cocaine (Liu et al, 2009). Although the modulatory role of CaMKIIα on prefrontal cortical D2 receptor substrates is not currently understood, the present evidence demonstrates for the first time, convergent increases in both total levels of CaMKIIα and increased D2 receptor expression following chronic opiate exposure. Furthermore, these molecular alterations corresponded to a loss in sensitivity of D2R receptor function in the modulation of opiate reward memory formation, dependent upon drug exposure state, consistent with previous reports linking CaMKIIα with D2R functional parameters, both in vitro and in vivo, in subcortical regions.
In addition, it is possible that increased intra-mPFC D2R and/or tCaMKIIα expression in the opiate chronic exposure/withdrawn state renders D2-CaMKII-dependent memory mechanisms resistant to antagonism by pharmacological manipulation. Increased expression of tCaMKIIα may also reflect the formation of a stronger, more stable opiate reward memory, as cortical CaMKIIα expression has been associated with the consolidation and permanence of classical conditioning memories (Frankland et al, 2001; Naskar et al, 2014). Increases to tCaMKIIα may also indicate a sensitized response to the exposure of an opiate-related cue (ie, the morphine environment). Given our findings of increased cortical D2R expression following chronic opiate exposure, it is of interest that previous reports have demonstrated decreased D2R function in subcortical brain regions involved in drug-reward processing. For example, heroin addicts display downregulated D2R levels in the striatum (Martinez et al, 2012; Wang et al, 1997), suggesting a hypo-function of mesolimbic inputs to striatal regions. Although we are not aware of any imaging studies demonstrating increased prefrontal cortical D2R expression levels in the opiate chronic exposure state, our findings may suggest that subcortical vs cortical DAergic transmission patterns are differentially altered in states of spontaneous withdrawal. Indeed, given that intra-BLA molecular and pharmacological DA function is opposite to that observed in the mPFC as a function of opiate exposure state, the present findings suggest important functional differences between cortical vs subcortical regulation of DA receptor function as a function of opiate exposure state.
The observed increase in mPFC pERK1 expression is consistent with previously reported increases in prefrontal cortical pERK following spontaneous withdrawal from chronic heroin self-administration (Edwards et al, 2009). Although intra-BLA ERK signaling has previously been shown to be necessary for opiate reward memory formation in the drug-naïve state (Lyons et al, 2013), the lack of behavioral effect of ERK inhibition in the mPFC remains consistent with data from Gholizadeh et al (2013), wherein acute opiate memory consolidation was shown to be ERK dependent in the BLA, but ERK independent in the mPFC. Whether changes to ERK activation or expression demonstrates a behaviorally relevant role in intra-mPFC opiate reward memory processing at other stages of memory processing (ie, later stage consolidation or retrieval) is still unknown. Alternatively, a significant increase in pERK1 activation following chronic heroin administration could render antagonism of ERK1 more difficult to achieve, and thus masking the behavioral effects of intra-mPFC ERK blockade. Finally, given that ERK1/2 is involved with many other molecular pathways, it is possible that the observed increase in pERK1 following chronic heroin exposure was mediated by an upstream change that does not involve the D1R, further suggested by our findings that increased expression in ERK1 levels did not correspond to any observable changes in the expression levels of intra-mPFC D1R.
Consistent with previous reports demonstrating a functional interplay between the BLA and mPFC during opiate reward memory processing (Gholizadeh et al, 2013; Sun and Laviolette, 2012), we found that simultaneous contralateral disconnection of intra-BLA ERK from intra-mPFC CaMKII signaling was sufficient to block opiate reward memory formation. In contrast, unilateral inhibition of ERK signaling was not sufficient for this effect. Given that we observed a functional switch in the roles of both the D2R and CaMKII signaling as a function of opiate exposure state, which occurred in the opposite direction to that reported previously in the BLA (Lyons et al, 2013), future studies are required to more fully characterize how intra-BLA ERK activity may regulate associative neuronal activity in the mPFC and vice versa. Furthermore, an investigation into how these functional switches in the role of D2R and CaMKII signaling may impact the functioning of circuitry related to the formation of more naturalistic rewards, such as sucrose, would be an interesting extension of these results. Nevertheless, the present findings further implicate the functional importance of BLA-mPFC interconnectedness, both in terms of DAergic transmission and molecular substrates linked to memory processing during the opiate addiction process.
Funding and Disclosure
The authors declare no conflict of interest.
References
Achterberg KG, Buitendijk GHS, Kool MJ, Goorden SMI, Post L, Slump DE et al (2014). Temporal and region-specific requirements of αCaMKII in spatial and contextual learning. J Neurosci 34: 11180–11187.
Andersen JM, Klykken C, Mørland J (2012). Long-term methadone treatment reduces phosphorylation of CaMKII in rat brain. J Pharm Pharmacol 64: 843–847.
Bechara A, Nader K, van der Kooy D (1995). Neurobiology of withdrawal motivation: evidence for two separate aversive effects produced in morphine-naïve versus morphine-dependent rats by both naloxone and spontaneous withdrawal. Behav Neurosci 109: 91–105.
Bilecki W, Zapart G, Ligeza A, Wawrzczak-Bargiela A, Urbański MJ, Przewłocki R (2005). Regulation of the extracellular signal-regulated kinases following acute and chronic opioid treatment. Cell Mol Life Sci 62: 2369–2375.
Bishop SF, Lauzon NM, Bechard MA, Gholizadeh S, Laviolette SR (2011). NMDA receptor hypofunction in the prelimbic cortex increases sensitivity to the rewarding properties of opiates via dopaminergic and amygdalar substrates. Cereb cortex 21: 68–80.
Bossert JM, Stern AL, Theberge FRM, Marchant NJ, Wang H-L, Morales M et al (2012). Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci 32: 4982–4991.
Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002). Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26: 321–352.
Chen Y, Jiang Y, Yue W, Zhou Y, Lu L, Ma L (2008). Chronic, but not acute morphine treatment, up-regulates alpha-Ca2+/calmodulin dependent protein kinase II gene expression in rat brain. Neurochem Res 33: 2092–2098.
Divsalar K, Meymandi MS, Afarinesh M, Zarandi MM, Haghpanah T, Keyhanfar F et al (2014). Serum biochemical parameters following heroin withdrawal: an exploratory study. Am J Addict 23: 48–52.
Doherty JM, Cooke BM, Frantz KJ (2013). A role for the prefrontal cortex in heroin-seeking after forced abstinence by adult male rats but not adolescents. Neuropsychopharmacology 38: 446–454.
Edwards S, Graham DL, Whisler KN, Self DW (2009). Phosphorylation of GluR1, ERK, and CREB during spontaneous withdrawal from chronic heroin self-administration. Synapse 63: 224–235.
Frankland P, O’Brien C, Ohno M (2001). α-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature 309–313.
Fricks-Gleason AN, Marshall JF (2011). Role of dopamine D1 receptors in the activation of nucleus accumbens extracellular signal-regulated kinase (ERK) by cocaine-paired contextual cues. Neuropsychopharmacology 36: 434–444.
Gholizadeh S, Sun N, De Jaeger X, Bechard MA, Coolen L, Laviolette SR (2013). Early versus late-phase consolidation of opiate reward memories requires distinct molecular and temporal mechanisms in the amygdala-prefrontal cortical pathway. PLoS One 8: e63612.
De Jaeger X, Bishop SF, Ahmad T, Lyons D, Ng GA, Laviolette SR (2013). The effects of AMPA receptor blockade in the prelimbic cortex on systemic and ventral tegmental area opiate reward sensitivity. Psychopharmacology (Berl) 225: 687–695.
Lai Y-T, Fan H-Y, Cherng CG, Chiang C-Y, Kao G-S, Yu L (2008). Activation of amygdaloid PKC pathway is necessary for conditioned cues-provoked cocaine memory performance. Neurobiol Learn Mem 90: 164–170.
Lauzon NM, Ahmad T, Laviolette SR (2012). Dopamine D4 receptor transmission in the prefrontal cortex controls the salience of emotional memory via modulation of calcium calmodulin-dependent kinase II. Cereb cortex 22: 2486–2494.
Laviolette SR, van der Kooy D (2003). Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry 8: 50–59 9.
Laviolette SR, van der Kooy D (2004). GABAA receptors signal bidirectional reward transmission from the ventral tegmental area to the tegmental pedunculopontine nucleus as a function of opiate state. Eur J Neurosci 20: 2179–2187.
Lintas A, Chi N, Lauzon NM, Bishop SF, Gholizadeh S, Sun N et al (2011). Identification of a dopamine receptor-mediated opiate reward memory switch in the basolateral amygdala-nucleus accumbens circuit. J Neurosci 31: 11172–11183.
Lintas A, Chi N, Lauzon NM, Bishop SF, Sun N, Tan H et al (2012). Inputs from the basolateral amygdala to the nucleus accumbens shell control opiate reward magnitude via differential dopamine D1 or D2 receptor transmission. Eur J Neurosci 35: 279–290.
Liu X-Y, Mao L-M, Zhang G-C, Papasian CJ, Fibuch EE, Lan H-X et al (2009). Activity-dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron 61: 425–438.
Liu Z, Liu X-D, Zhang J-J, Yu L-C (2012a). Increases in αCaMKII phosphorylated on Thr286 in the nucleus accumbens shell but not the core during priming-induced reinstatement of morphine-seeking in rats. Neurosci Lett 526: 39–44.
Liu Z, Zhang J-J, Liu X-D, Yu L-C (2012b). Inhibition of CaMKII activity in the nucleus accumbens shell blocks the reinstatement of morphine-seeking behavior in rats. Neurosci Lett 518: 167–171.
Lu L, Zeng S, Liu D, Ceng X (2000). Inhibition of the amygdala and hippocampal calcium/calmodulin-dependent protein kinase II attenuates the dependence and relapse to morphine differently in rats. Neurosci Lett 291: 191–195.
Lucas M, Frenois F, Cador M, Le Moine C (2012). Remodeling of the neuronal circuits underlying opiate-withdrawal memories following remote retrieval. Neurobiol Learn Mem 97: 47–53.
Lyons D, de Jaeger X, Rosen LG, Ahmad T, Lauzon NM, Zunder J et al (2013). Opiate exposure and withdrawal induces a molecular memory switch in the basolateral amygdala between ERK1/2 and CaMKIIα-dependent signaling substrates. J Neurosci 33: 14693–14704.
Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A et al (2012). Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry 71: 192–198.
Morón JA, Gullapalli S, Taylor C, Gupta A, Gomes I, Devi LA (2010). Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology 35: 955–966.
Nader K, Harrington F, Bechara A, van der Kooy D (1994). Neuroleptics block high but not low dose heroin place preferences: further evidence for a two system model of motivation. Behav Neurosci 108: 1128–1138.
Narita M, Matsumura Y, Ozaki S, Ise Y, Yajima Y, Suzuki T (2004). Role of the calcium/calmodulin-dependent protein kinase ii (CaMKII) in the morphine-induced pharmacological effects in the mouse. Neuroscience 126: 415–421.
Naskar S, Wan H, Kemenes G (2014). pT305-CaMKII stabilizes a learning-induced increase in AMPA receptors for ongoing memory consolidation after classical conditioning. Nat Commun 5: 3967.
Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN et al (2012). Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Mol Psychiatry 17: 85–98.
Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press: : Amsterdam.
Pelloux Y, Murray JE, Everitt BJ (2013). Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur J Neurosci 38: 3018–3026.
Sun N, Chi N, Lauzon N, Bishop S, Tan H, Laviolette SR (2011). Acquisition, extinction, and recall of opiate reward memory are signaled by dynamic neuronal activity patterns in the prefrontal cortex. Cereb cortex 21: 2665–2680.
Sun N, Laviolette SR (2012). Inactivation of the basolateral amygdala during opiate reward learning disinhibits prelimbic cortical neurons and modulates associative memory extinction. Psychopharmacology (Berl) 222: 645–661.
Valjent E, Pagès C, Hervé D, Girault J-A, Caboche J (2004). Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci 19: 1826–1836.
Wang G, Volkow N, Fowler J (1997). Dopamine D 2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology 16: 174–182.
Zhang S, Xie C, Wang Q, Liu Z (2014). Interactions of CaMKII with dopamine D2 receptors: roles in levodopa-induced dyskinesia in 6-hydroxydopamine lesioned Parkinson’s rats. Sci Rep 4: 6811.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (MOP 123378), the Natural Science and Engineering Research Council of Canada (NSERC), and an Ontario Graduate Scholarship to LGR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosen, L., Zunder, J., Renard, J. et al. Opiate Exposure State Controls a D2-CaMKIIα-Dependent Memory Switch in the Amygdala-Prefrontal Cortical Circuit. Neuropsychopharmacol 41, 847–857 (2016). https://doi.org/10.1038/npp.2015.211
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.211
This article is cited by
-
Cannabidiol Modulates Fear Memory Formation Through Interactions with Serotonergic Transmission in the Mesolimbic System
Neuropsychopharmacology (2016)