Abstract
A new pharmacokinetic approach treating cocaine addiction involves rapidly metabolizing cocaine before it reaches brain reward centers using mutated human butyrylcholinesterase (BChE) or cocaine hydrolase (CocH). Recent work has shown that helper-dependent adenoviral (hdAD) vector-mediated plasma CocH reduced the locomotor-activating effects of cocaine and prevented reinstatement of cocaine-seeking behavior up to 6 months in rats. The present study investigated whether hdAD-CocH could decrease ongoing intravenous cocaine (0.4 mg/kg) self-administration. The hdAD-CocH vector was injected into self-administering rats, and after accumulation of plasma CocH, there was a dramatic reduction in cocaine infusions earned under a fixed ratio 1 schedule of reinforcement that lasted for the length of the study (>2 months). Pretreatment with the selective BChE and CocH inhibitor iso-OMPA (1.5 mg/kg) restored cocaine intake; therefore, the decline in self-administration was likely due to rapid CocH-mediated cocaine metabolism. Direct measurements of cocaine levels in plasma and brain samples taken after the conclusion of behavioral studies provided strong support for this conclusion. Further, rats injected with hdAD-CocH did not experience a deficit in operant responding for drug reinforcement and self-administered methamphetamine (0.05 mg/kg) at control levels. Overall, these outcomes suggest that viral gene transfer can yield plasma CocH levels that effectively diminish long-term cocaine intake and may have potential treatment implications for cocaine-dependent individuals seeking to become and remain abstinent.
Similar content being viewed by others
INTRODUCTION
A high prevalence of cocaine addiction in the United States results in considerable morbidity and mortality for cocaine users and enormous economic costs to society. Currently, no sustainable treatment is available. This study examined a potentially successful therapy involving long-term gene transfer of an enzyme that rapidly hydrolyzes cocaine to inactive metabolites. The enzyme, a ‘cocaine hydrolase’ (CocH) derived by multiple mutations of human butyrylcholinesterase (BChE; Zheng et al, 2008; Xue et al, 2013), is expected to reduce drug access to brain. If CocH were sustained at adequate levels, it might aid users who desire to become abstinent. We have carried out several studies testing the efficacy of CocH treatment in rodent models. These began with a focus on relapse, ie, reinstatement of cocaine seeking by rats in forced abstinence after training on cocaine self-administration. Rats treated i.v. with a purified version of CocH protein, an hour before i.p. priming with cocaine, failed to reinstate their drug-seeking behavior (Brimijoin et al, 2008). Delivery of CocH by viral gene transfer increased cocaine metabolism several thousand-fold in rats and mice (Gao et al, 2005, Gao and Brimijoin 2004, 2006), and animals showed no locomotor response to normal stimulatory doses of cocaine (Carroll et al, 2012). In fact, a single treatment of CocH delivered by a helper-dependent adenoviral (hdAD) vector was able to block cocaine-primed reinstatement for 6 months or more (Anker et al, 2012b), yet d-amphetamine induced robust reinstatement. Thus, the CocH vector had no debilitating motivational effects, and it was specific to cocaine’s reinforcement value.
More recent gene transfer experiments using large doses of viral vector in mice have succeeded in generating plasma CocH for periods approaching 2 years at levels that prevent all observable reactions to cocaine in near-lethal doses (Geng et al, 2013). Because these treatments elicited no apparent toxicity, we were encouraged to explore the effects of high-dose CocH gene transfer in rats maintaining robust self-administration of i.v. cocaine over a 2-month period. The main purpose of the present study was to examine effects on cocaine self-administration, and long-term suppression of cocaine seeking was the goal. A secondary purpose was to establish that beneficial effects on cocaine seeking are specifically due to enzymatic activity. We tested two linked hypotheses: (1) vector-mediated CocH would abolish ongoing cocaine self-administration that would be reversed following systemic administration of iso-OMPA, a selective inhibitor of BChE and CocH, and (2) vector-mediated CocH would not suppress methamphetamine (a non-hydrolysable substrate) self-administration.
MATERIALS AND METHODS
Animals
Thirty-two adult female Wistar rats, ranging in weight from 250 to 300 g, were purchased from Harlan (Indianapolis, IN). Female rats were used because they exhibit higher rates of cocaine self-administration than male rats (Carroll and Anker, 2010; Anker and Carroll, 2011). For at least 3 days following arrival, rats were pair-housed in polycarbonate cages in a colony room where they had ad libitum access to rat chow (Teklad 2018, Harlan) and water. After acclimation, they were implanted with chronic indwelling jugular catheters and singly housed in operant conditioning chambers for the remainder of the study. Under these conditions, they had ad libitum access to water and were fed 16 g of chow after session to maintain them at 85% free-feeding body weight. All rodent holding rooms were maintained at 24 °C and at 40–50% humidity under a light/dark cycle (12/12-h) with room lights on at 06 00 hours. The experimental protocol (1008A87756) was approved by the University of Minnesota Institutional Animal Care and Use Committee. The experiment was conducted in compliance with the Guide for the Care and Use of Animals (National Research Council, 2011), and all laboratory facilities were accredited by the American Association for the Accreditation of Laboratory Animal Care.
Apparatus
Rats were tested in eight-sided custom-built operant conditioning chambers enclosed in wooden sound-attenuating boxes fitted with a ventilation fan. Operant conditioning chambers had alternating clear polycarbonate plastic and stainless steel walls, two response levers (H21-03R, Coulbourn Instruments, Whitehall, PA, USA), two sets of multi-colored stimulus lights (one above each response lever; H11-02R, Coulbourn Instruments), one house light (4.76W; H11-01R, Coulbourn Instruments), water bottle, and chow receptacle.
During cocaine self-administration sessions, responding on the active/drug-paired lever activated the syringe pump (PHM-100, MedAssociates, St Albans, VT, USA) to deliver drug infusions through a swivel-tether (375/22PS, Instech, Plymouth Meeting, PA, USA, C313CS-MN, PlasticsOne, Roanoke, VA, USA) infusion system that was attached to an infusion harness (CIH95AB, Instech) worn by the rat. Data collection and programming were conducted using PC computers with a Med-PC interface (MedAssociates).
Drugs
Cocaine HCl and methamphetamine were obtained from Research Triangle Institute (Research Triangle Park, NC, USA) and dissolved in sterile saline at concentrations of 1.6 and 0.2 mg/ml, respectively. Heparin (5 USP/ml) was added to cocaine and methamphetamine solutions to prevent catheter occlusion from thrombin accumulation. The flow rate of each infusion was 0.025 ml/s, and the duration of pump activation (1 s/100 g of body weight) was adjusted weekly to provide a 0.4 mg/kg/infusion cocaine dose or 0.05 mg/kg/infusion methamphetamine dose throughout self-administration testing. Because of the length of the within-subjects procedure and the goal of investigating multiple doses of hdAD-CocH, we examined only one dose of cocaine and methamphetamine. The doses of cocaine and methamphetamine used were chosen because they are readily administered and maintain similar levels of self-administration (Carroll et al, 2011; Anker et al, 2012a). Iso-OMPA (tetra isopropyl pyrophosphoramide, Sigma-Aldrich, St Louis, MO, USA), an irreversible organophosphate anticholinesterase selective for BChE and BChE-mutants like CocH, was dissolved in sterile saline at a concentration of 1 mg/ml and delivered by i.p. injection at a dose of 1.5 mg/kg.
Viral Vector Encoding CocH
Rats in the CocH vector-treated group were injected with a hdAD vector (Parks et al, 1996) that contained cDNA for CocH under regulation by a human ApoE hepatic control region, with a bovine growth hormone polyadenylation sequence cloned into a derivative of the p28lacZ hdAD-backbone plasmid. This vector was propagated using the AdNG163 helper virus, and particle titers were determined by optical density at 260 nm. Helper virus contamination, determined by plaque assay on HEK-293 cells, was approximately 0.2%. Rats were treated with dexamethasone HCl 12–15 and 2 h (10 mg/kg, i.p.) before vector injection and again 24 h (5 mg/kg, i.p.) after vector injection to mildly suppress the immune system and facilitate vector transduction. Viral vector delivery was accomplished by rapid injection of a solution containing either 1011 (CocH vector × 1, N=7) or 1012 (CocH vector × 10, N=13) viral particles through the tail vein in an initial volume of 1 ml following by 0.2 ml of sterile saline. Control rats (N=12) were injected identically with the same volume of saline.
Catheterization Surgery
Rats were implanted with chronic indwelling jugular catheters following methods previously described (Carroll and Boe, 1982; Zlebnik et al, 2010). Following the surgical procedure, rats were allowed 3 days to recover with administration of antibiotics (enrofloxacin, 10 mg/kg, s.c.) and analgesics (buprenorphine, 0.05 mg/kg, s.c.; ibuprofen, 15 mg/kg, p.o.). After surgery and until the remainder of the experiment, rats wore an infusion harness (CIH95AB, Instech) and tether (C313CS-MN, PlasticsOne) and were chronically housed in operant conditioning chambers where they underwent daily i.v. drug self-administration sessions. Catheters were flushed with a solution (0.3 ml, i.v.) of heparinized saline (20 USP/ml) and cefazolin (10.0 mg/ml) daily to prevent catheter blockage and infection. Weekly, rats were weighed, and catheter patency was verified by injecting a 0.1-ml solution containing ketamine (60 mg/kg), midazolam (3 mg/kg), and saline into the catheter. If loss of the righting reflex did not manifest, a second catheter was implanted in the left jugular vein, and the experiment was resumed after a 3-day recovery period. Including all groups, 10 rats (control, N=3; CocH vector × 1, N=3; CocH vector × 10, N=4) received a second catheter implant.
Blood Collection and Weekly Measurement of Plasma CocH Activity
Rats were briefly restrained in a clear plastic tube, and the free end of the tail was submerged in warm water to increase visibility of the lateral tail vein. A 23-G butterfly infusion needle was inserted into the vein, approximately one-third the distance from the tail tip, and gently suctioned until up to 0.2 ml of blood was obtained. All blood samples were centrifuged for 15 min at 8000 g in serum separator tubes (Becton Dickenson, Franklin Lakes, NJ, USA), and plasma was stored at −20 °C before being analyzed for active CocH enzyme levels. CocH activity in 50 μl supernatant aliquots was assayed by incubating for 30 min with 3H cocaine (18 μM, except for substrate kinetics) and measuring liberated 3H-benzoic acid (Brimijoin et al, 2002).
Cocaine Determinations in Plasma and Brain
Following the conclusion of the behavioral procedures, the ability of CocH to rapidly reduce plasma and brain concentrations of cocaine was examined in a terminal procedure. Rats (CocH vector × 10, N=8; control, N=5) were first anesthetized with pentobarbital (50 mg/kg, i.p., ‘Sleepaway,’ Zoetis, Kalamazoo, MI, USA), the chest was opened to expose the heart, and a 23-G needle was inserted into the tail vein. 3H-cocaine (0.4 mg/kg) was then rapidly injected, followed by a 0.5 ml flush of isotonic saline. Thirty seconds later, the apex of the left ventricle was nicked, and 1 ml of blood was transferred to a tube with 10 μl of the irreversible cholinesterase inhibitor ‘DFP’ (di-isopropylfluorophosphate, 10−2 M). Immediately afterward, the rat was perfused with 70 ml of saline with DFP at 10−5 M, the skull was opened, and the brain was removed onto dry ice. Blood samples were centrifuged 2500 g for 5 min to obtain plasma. Brain samples were homogenized in 10 mM sodium phosphate pH 7.5 with 0.1% Tween 80 and DFP (10−5 M), mixed well, and centrifuged for 5 min at 2500 g to obtain supernatant for assay. For determinations of radiolabeled cocaine, 200 μl of plasma or 200 μl of brain supernatant were then mixed with 1 M Na2CO3 and 4 ml of toluene fluor as previously described by Brimijoin et al (2008).
PROCEDURE
Cocaine Self-Administration Pre- and Post-CocH Vector Injection
Cocaine (0.4 mg/kg/infusion) self-administration training occurred during daily 2-h sessions (0900–1100 hours). At the start of session, the house light located at the top of the cage illuminated, and each response on the active lever produced one intravenous infusion of cocaine (fixed-ratio 1 schedule of reinforcement) and turned on the set of stimulus lights above that lever for the duration of the infusion (2.5–3.5 s). Responses on the inactive lever also illuminated its corresponding stimulus lights but otherwise had no scheduled consequences. Initially, a small amount of peanut butter and three experimenter-delivered active lever presses/infusions were delivered at the start of each session. Once a rat began self-administering at least 20 infusions/session, peanut butter was discontinued and the three free experimenter-delivered active lever presses were phased out over the next three sessions. Following acquisition of cocaine self-administration, rats were allowed to continue self-administering cocaine for at least 10 consecutive sessions before CocH-encoded hdAD vector or control (saline) injection (Figure 1). The hdAD vector was delivered between 1100 and 1300 hours, and rats were allowed to resume cocaine self-administration the following day. For the next 30 days, daily cocaine self-administration was monitored, and blood was collected from the lateral tail vein weekly to determine plasma levels of CocH.
Timeline of experimental interventions. Arrows indicate investigator-delivered injections.
Suppression of CocH Activity by Iso-OMPA
After at least 30 days of opportunity for cocaine self-administration following hdAD vector injection, plasma levels of transduced CocH were deliberately suppressed by daily pretreatment with the BChE-selective inhibitor iso-OMPA in a within-subjects design (Figure 1). As there was no significant therapeutic effect of plasma CocH on cocaine self-administration in the CocH vector × 1 group (1011 viral particles/rat), this experimental manipulation was administered only to the CocH vector × 10 (1012 viral particles/rat) and control rats. After 20 days of stable self-administration conditions, rats were pretreated 12 h before their session with either iso-OMPA (1.5 mg/kg, i.p.) or saline (equivalent volume, i.p.) injections. Because the CocH vector × 10 rats had extinguished responding by this time (stable near-zero levels of cocaine self-administration following CocH-encoded hdAD vector injection), three experimenter-administered active lever presses/infusions (i.v. ‘priming’ injections) were delivered to instigate self-administration at the start of these sessions in both groups. Subsequently, rats were pretreated with saline for three consecutive sessions, followed by another four sessions to recover baseline responding in the event that the priming injections delivered at the beginning of the saline pretreatment sessions promoted a significant return to self-administration, and then they were pretreated with iso-OMPA for the next eight consecutive sessions. This length of iso-OMPA treatment was chosen in light of preliminary trials suggesting that 3 days of iso-OMPA pretreatment was not enough to fully inhibit CocH in rats with high plasma levels of the protein (unpublished observations). Plasma CocH was sampled daily after session (between 1100 and 1300 hours) during iso-OMPA pretreatment and for 8 days following its termination.
Methamphetamine Self-Administration
After the iso-OMPA treatments, cocaine self-administration was allowed to stabilize and return to baseline during a 10- to 14-day period. Subsequently, cocaine solutions were replaced with methamphetamine (0.05 mg/kg/infusion) solutions for the CocH vector × 10 and control groups, and rats were allowed to self-administer methamphetamine under identical conditions for the next 8 days. Although methamphetamine is a psychostimulant similar to cocaine, it is not a substrate of CocH, and it was predicted to maintain high levels of self-administration in both CocH vector × 10 rats and controls. Previous work demonstrated that d-amphetamine reinstated cocaine-seeking behavior suppressed by CocH (Brimijoin et al, 2008).
Data Analysis
The primary dependent measures were drug (eg, cocaine, methamphetamine) infusions, CocH plasma levels (U/ml), and 3H-cocaine plasma and brain levels (μg/ml). For cocaine self-administration before and after vector injection, data were grouped into 10-day blocks to reduce daily variability and the number of post-hoc contrasts. They were then analyzed by two-factor mixed analyses of variance (ANOVA) with treatment group (CocH vector × 1 vs CocH vector × 10 vs control) as the between-subjects factor and blocks of days as the repeated measure. For the iso-OMPA treatment experiment and the methamphetamine substitution experiment, data were averaged across the treatment or substitution periods and analyzed by two-factor ANOVA. Following significant interactions, post hoc tests were performed with the Tukey–Kramer procedure, and results were considered significant if p<0.05. Statistical analyses were performed using GB Stat (Dynamic Microsystems, Silver Spring, MD, USA).
RESULTS
Reduction in Cocaine Self-Administration Following CocH Vector Delivery
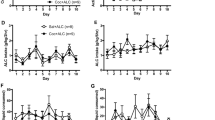
Daily cocaine self-administration before and after CocH vector delivery is displayed in Figure 2. Results of the ANOVA revealed significant main effects of group (F2, 127=12.18, p<0.01) and a significant group × block interaction (F6,127=22.67, p<0.0001). Post hoc analyses showed that, for the 10-day block before CocH vector injection, all rats earned a similar number of cocaine infusions. After vector delivery, the CocH vector × 1 group had a considerable increase in their daily infusions (vs controls, p<0.01; vs baseline, p<0.01) that persisted for a minimum of 20 days. In contrast, for the 10-day block following vector delivery, the CocH vector × 10 rats at first increased their infusions relative to controls and then drastically reduced their infusions to near-zero levels (vs CocH vector × 1, p<0.01; vs baseline, p<0.01). Outside the periods of experimental manipulation (ie, iso-OMPA treatment, methamphetamine substitution), cocaine self-administration remained at low levels in the CocH vector × 10 rats for the remainder of the study (>9 weeks).
Mean (±SEM) cocaine infusions before and after delivery of CocH vector or vehicle. CocH vector × 1 rats notably increased their infusions relative to controls (**p<0.01) and baseline (@p<0.01) following hdAD-CocH vector injection. Although the CocH vector × 10 rats increased their infusions for the 10 days following vector delivery, their infusions subsequently decreased to near-zero levels and were significantly lower than their infusions at baseline (@p<0.01) and lower than that of CocH vector × 1 rats (**p<0.01).
Mean CocH plasma levels (expressed in U/ml of rat plasma; 1 U=1 μmol cocaine hydrolyzed/min) were stable during weekly monitoring (Table 1), and rats in the CocH vector × 10 group had 30-fold more plasma CocH than rats in the CocH vector × 1 group (main effect: F1,79=18.86, p<0.01). For comparison, CocH activity measured in plasma from saline-treated controls and pre-hdAD vector injection samples from rats in the CocH vector-treated groups was approximately 0.0001 U/ml. Therefore, even at week 1, hdAD vector treatment raised this activity by a factor of 3000 (CocH vector × 1 group) to 100 000 (CocH vector × 10 group).
BChE Inhibitor Iso-OMPA Rescues Cocaine Self-Administration in CocH Vector Rats
Cocaine self-administration following saline or iso-OMPA pretreatment is shown in Figure 3a. The mean number of infusions earned following saline pretreatment was compared with the number earned following iso-OMPA pretreatment in a two-factor ANOVA, and results indicated both a significant main effect of treatment (F1, 39=5.21, p<0.05) and a significant group × treatment interaction (F1, 39=10.02, p<0.01). During the period of saline pretreatment, CocH vector × 10 rats had lower cocaine self-administration than control rats (NS). However, iso-OMPA treatment conditions resulted in a recovery of cocaine self-administration in the CocH group (p<0.01) and elicited comparable levels of self-administration in CocH rats and control rats.
Mean (±SEM) cocaine infusions and CocH plasma levels (U/ml) during treatment with the BChE inhibitor iso-OMPA or vehicle. (a) Pretreatment with iso-OMPA significantly increased cocaine self-administration (#p<0.01) in the CocH vector rats, bringing their cocaine infusions to control levels. Shown in the bar graph are the mean infusions during the saline and iso-OMPA treatment periods. (b) Plasma CocH levels decreased during iso-OMPA treatment (gray circles) and remained significantly lower than pre-iso-OMPA treatment levels (Session ‘0’) for 7 days following cessation of treatment (*p<0.05).
Figure 3b depicts daily CocH plasma levels before, during, and after iso-OMPA treatment. Post-hoc analyses following a significant F-ratio (F2, 29=7.90, p<0.005) demonstrated that mean plasma levels of CocH were substantially reduced (∼ 90%) by daily iso-OMPA injections (p<0.01). CocH levels partially recovered following the discontinuation of the treatment, but they remained significantly lower (p<0.05) than before the inhibitor was administered. Despite the incomplete recovery, cocaine self-administration returned to very low levels in the CocH vector × 10 rats (Figure 4), with CocH rats earning significantly fewer infusions than control rats (t12=4.96, p<0.0005).
Mean (±SEM) cocaine or methamphetamine infusions. Following iso-OMPA treatment, self-administration stabilized, and CocH rats had significantly lower self-administration of cocaine than control rats (**p<0.01). However, when cocaine was substituted with methamphetamine under identical conditions, CocH vector and control rats maintained similar levels of self-administration.
Methamphetamine Maintains Self-Administration in CocH Vector Rats
The CocH vector × 10 group and the control group were tested with methamphetamine in order to evaluate responding for a non-hydrolysable psychostimulant drug. Despite differential levels of cocaine self-administration (p<0.01), CocH vector × 10 and control rats self-administered similar levels of methamphetamine (Figure 4). The mean number of infusions earned during methamphetamine (0.05 mg/kg) access did not differ between the groups.
Relation between CocH Activity and Cocaine Levels in Plasma and Brain
Table 2 presents cocaine levels from the controls (N=5) and from CocH vector × 10 rats, stratified by their concurrent level of CocH activity as ‘low CocH’ (0.3–3.3 U/ml, N=4) and ‘high CocH’ (11.3–28 U/ml, N=4). The data show that plasma cocaine was reduced by more than 98% in both CocH vector-treated subgroups as compared with controls. In contrast, brain cocaine levels were strongly dependent on CocH activity such that brains from ‘low CocH’ rats accumulated 51% as much cocaine as the controls (NS), but brains from ‘high CocH’ rats accumulated less than 2% as much (p<0.005).
DISCUSSION
The present findings collectively demonstrate that a single injection of hdAD-CocH vector at very high dose can generate and sustain levels of CocH that virtually eliminate ongoing responding for i.v. cocaine at the 0.4 mg/kg unit dose in rats. The simplest explanation for this effect is that the circulating enzyme reduces the amplitude and the rate of rise of an i.v. cocaine ‘spike’ and substantially degrades its reinforcement value. Given that enzymatic hydrolysis cannot be instantaneous, and that the cocaine injections were delivered in rapid pulses through a catheter in the vena cava, near the right atrium, this outcome is remarkable.
A consistent feature of the CocH vector effect on self-administration was an initial increase in operant responding, which was transient in the high-dose group (CocH vector × 10) but prolonged in the low-dose group (CocH vector × 1). The rats that received the lower dose of hdAD-CocH vector (1011 viral particles/rat) and produced lower plasma levels of CocH showed elevated cocaine self-administration for most of the subsequent 30-day testing period. However, in animals that received higher dose CocH vector (1012 viral particles/rat), responding and cocaine infusions both began rising 4 days after gene transfer, reached a peak threefold above baseline at 7 days, and then fell to zero after 10 days. These time points corresponded roughly to plasma CocH levels of 1, 10, and 30 U/ml (data not shown).
Although we could not track ‘real time’ plasma and brain cocaine in these animals during the self-administration phase, we can draw useful inferences from the terminal experiment with radiolabeled cocaine. In addition to five untreated controls, this experiment was conducted in eight rats given vector at the 1012 particles/rat dose (CocH vector × 10), including four rats whose CocH expression remained very high (>10 U/ml), and four others whose expression had dropped to levels characteristic of the CocH vector × 1 rats (∼1 U/ml). These data provide a basis for estimating the cocaine levels that were achieved after a single delivered i.v. cocaine injection. In particular, the results on cocaine in blood and brain imply that the lowest enzyme level was probably sufficient to cause a drop in net cocaine delivery that reduced its reinforcement value noticeably but not greatly, thus inducing the rats to compensate by more lever pressing for more infusions. On the other hand, the highest level of CocH expression was accompanied by a drastic reduction of brain cocaine under tested conditions. It seems quite reasonable that the residual 2% accumulation should be inadequate to sustain responding for reinforcement.
These results are in accord with those from our recent study on mice given i.v. CocH protein (Brimijoin et al, 2013), in which drug and enzyme levels were examined in plasma and brain 5 min after i.v. 3H-cocaine administration (0.1 mg/kg). Those data showed that a 1 U/ml level of CocH activity decreased 3H-cocaine concentrations by >95% in plasma and >50% in brain (higher levels of CocH were not attempted). Although there are substantial differences between rats and mice, the enzyme action on circulating cocaine should depend similarly on the concentrations of these two molecules. Therefore, in light of the available information, the persistently high level of cocaine self-administration in the CocH vector × 1 group, whose enzyme activities ranged from 0.3 to 1 U/ml is also consistent with our previous work demonstrating potentiated cocaine self-administration following acute i.v. injection of CocH protein (Carroll et al, 2011). This interpretation is more probable than one based on ‘increased motivation,’ for which there is no plausible mechanism, and which would not explain the ensuing collapse of drug-taking behavior. The transient spike of lever pressing for the CocH vector × 10 group should be viewed in the same way. On the way to their peak levels of CocH, the rats passed through the 0.3–1 U/ml range between days 3 and 7, and their lever pressing activity rose accordingly. By 14 days, however, they reached average plasma CocH levels of 30–45 U/ml that in vitro would eliminate >99% of a 1 μM cocaine solution in less than 15 s (Gao et al, 2010). Therefore, the lever-pressing pattern between days 7 and 14 likely represents ‘extinction responding’ in the context of vanishing cocaine reinforcement as plasma CocH activity escalated. Also worth comment, but difficult to explain, is a modest decrease in responding during the first 5 days after CocH vector treatment. As this decrease was only prominent in the CocH vector × 1 group it is difficult to explain in terms of non-specific CocH vector effects (eg, transient malaise), and we offer no speculation on the mechanism.
The effects of the enzyme inhibitor, iso-OMPA, make it clear that the vector-induced suppression of responding for cocaine reinforcement in the CocH vector × 10 group depended on accelerated cocaine removal. At the moderate doses used here this irreversible inhibitor is reasonably selective for BChE (Austin and Berry, 1953), from which CocH is derived (Pan et al, 2005; Sun et al, 2002; Zheng et al, 2008). Although it will affect other enzymes, such as carboxyl esterase (Grubic et al, 1988), it has little effect on acetylcholinesterase (AChE) and therefore spares cholinergic synapses in skeletal muscle, brain, and autonomic nerve endings (Mesulam, 2003). Thus, it was possible to eliminate >95% of the plasma CocH activity without observable changes in motor function. The resulting loss of cocaine hydrolysis appeared to restore the reinforcement value of drug as it was associated with renewed responding. It is particularly striking that this responding began to decline immediately after iso-OMPA dosing ended and ceased within 5 days, coinciding with restored CocH levels generated by ongoing vector-driven synthesis of the enzyme as its inhibitor was metabolized and eliminated.
Substitution of methamphetamine for cocaine provided a test of specificity for the vector treatment and its effect on responding for drug delivery. These two stimulants have different mechanisms of action at the cellular and molecular level but they tend to affect dopaminergic and adrenergic synapses in a similar manner (Johanson and Fischman, 1989; Seiden et al, 1993). Furthermore, their immediate behavioral effects, including enhanced locomotor activity are also similar (Berridge, 2006). However, methamphetamine is not an ester compound, and its metabolic inactivation differs radically from that of cocaine. Rather than being a substrate for circulating enzymes, amphetamines require metabolism by mitochondrial monoamine oxidase and/or hepatic cytochrome P450 systems, particularly CYP2D6 (Tucker et al, 1994). Hence, an esterase such as CocH will not affect availability of methamphetamine and access to targets in the brain. On the other hand, if rats given hdAD-CocH vector and iso-OMPA became less sensitive to stimulants in general, by some unknown mechanism, we would have expected decreased responding for methamphetamine as well as cocaine. As responding for methamphetamine was well maintained, we conclude that CocH acts selectively and specifically to eliminate cocaine reward value by hydrolyzing this ester drug into its virtually inactive metabolites, benzoic acid and ecgonine methyl ester (Crumb and Clarkson, 1992; Inaba, 1989).
It is encouraging that the high levels of transduced enzyme were not accompanied by overt signs of toxicity. Thus, the current findings are in line with our recent safety and toxicity study on mice (Murthy et al, 2013). That study also drove CocH transduction with very high doses of hdAD vector (1013 viral particles/mouse) or adeno-associated viral vector (AAV; 3 × 1014 viral genomes/kg). And it identified no deficits in motor performance, no sign of tissue damage in liver, muscle, or heart, and no effect on maze memory and learning, either with hdAD or AAV. It is not likely that human recipients could tolerate equivalent doses of viral vector, but a large body of evidence supports the idea that BChE in native and mutant forms is itself benign even at very high levels (Weber et al, 2011).
The potential for metabolic ‘drug–drug interactions’ after BChE gene transfer deserves brief consideration at this stage. It has been recognized for decades that BChE has an important role in metabolizing some clinical agents, notably, certain drugs used as inter-operative muscle-relaxants during abdominal surgery. The classic example is succinylcholine, which normally acts transiently but persists in patients with a deficiency or an ‘atypical allele’ of BChE, who may then require prolonged artificial respiration (Kalow, 2004). Mivacurium is another commonly used muscle relaxant whose short duration of action depends on BChE (Soliday et al, 2010). Patients expressing BChE at the levels attained in our rats would probably show no response at all to such drugs, and if muscle relaxation is surgically required they might need BChE-resistant agents with longer than optimal half-lives. An alternative solution might be to begin with a low dose of iso-OMPA (as used here), or a reversible and BChE-selective anti-cholinesterase such as ethopropazine, before giving the muscle relaxant. In any case, however, present information provides no reason to anticipate that an excess of BChE will directly induce toxicity or convert clinically useful drugs into toxic metabolites.
When CocH gene transfer is attempted in human subjects, the levels of enzyme expression will likely be lower than those achieved here. However, it is not clear that complete elimination of cocaine reinforcement is essential for a therapeutic effect. Although a ‘subthreshold’ dose of CocH elevated cocaine self-administration in rats (ie, CocH vector × 10 group), this effect was seen under artificial conditions of unlimited access to cocaine infusions under a simple FR 1 schedule of reinforcement. The progressive ratio schedule is more challenging, and our prior work demonstrated that acute i.v. injections of albu-CocH attenuated progressive ratio responding at doses that elevated FR 1 responding for cocaine (Carroll et al, 2011). Therefore, even lower levels of CocH activity may ultimately weaken motivation for cocaine when drug is not freely available. An extended duration of enzyme expression is a major advantage of gene transfer. The useful life of CocH gene therapy will depend on the class of viral vector and its serotype, among other important parameters. The vectors used here are non-integrating and do not insert themselves into chromosomal DNA. Hence, they are expected to persist only for the life of the host cells, mainly hepatocytes in the present case. But transduction in mice has lasted 2 years and more (Geng et al, 2013) and it is reasonable to expect similar or longer expression in humans.
It is premature to propose specific plans for translating this treatment to humans but we can identify issues that need consideration. Our initial work with nonhuman primates on demanding cocaine-reinforcement schedules has again revealed attempts to surmount the effects of rapid cocaine breakdown before drug-seeking behavior extinguishes. A treatment-seeking user might respond similarly. Therefore, early trials should be conducted with close medical supervision, possibly on an inpatient basis with a view toward generating new models for implementation in a context of safety in humans.
CONCLUSIONS AND FUTURE DIRECTIONS
The results of the present investigation confirm that it is possible to shut down ongoing cocaine self-administration in rodents by delivering and sustaining high levels of an enzyme that metabolizes this drug. As a proof of principle, this study demonstrates that gene transfer of protein therapeutics has the potential to be highly successful in treating cocaine addiction. Similar experiments with nonhuman primates are now beginning. Early results show that it is possible to achieve plasma CocH levels in Rhesus macaques that are comparable to those in our lower dose CocH vector rats, and without signs of distress or changes in circulating biomarkers for liver toxicity. Continued success along these lines, particularly if it brings mounting evidence for safety, may bring this treatment to the verge of a human trial. In the meantime, we remain mindful that gene transfer of a safe but highly active enzyme could be combined with a current generation anti-cocaine vaccine to offer a truly robust treatment for cocaine abuse.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Anker JJ, Baron TR, Zlebnik NE, Carroll ME (2012a). Escalation of methamphetamine self-administration in adolescent and adult rats. Drug Alcohol Depend 124: 149–153.
Anker JJ, Brimijoin S, Gao Y, Geng L, Zlebnik NE, Parks RJ et al (2012b). Cocaine hydrolase encoded in viral vector blocks the reinstatement of cocaine seeking in rats for 6 months. Biol Psychiatry 71: 700–705.
Anker JJ, Carroll ME (2011). Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Topics Behav Neurosci 8: 73–96.
Austin L, Berry WK (1953). Two selective inhibitors of cholinesterase. Biochem J 54: 695–700.
Berridge CW (2006). Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology 31: 2332–2340.
Brimijoin S, Gao Y, Anker JJ, Gliddon LA, Lafleur D, Shah R et al (2008). A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology 33: 2715–2725.
Brimijoin S, Orson F, Kosten TR, Kinsey B, Shen XY, White SJ et al (2013). Anti-cocaine antibody and butyrylcholinesterase-derived cocaine hydrolase exert cooperative effects on cocaine pharmacokinetics and cocaine-induced locomotor activity in mice. Chem Biol Interact 203: 212–216.
Brimijoin S, Shen ML, Sun H (2002). Radiometric solvent-partitioning assay for screening cocaine hydrolases and measuring cocaine levels in milligram tissue samples. Anal Biochem 309: 200–205.
Carroll ME, Anker JJ (2010). Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav 58: 44–56.
Carroll ME, Boe IN (1982). Increased intravenous drug self-administration during deprivation of other reinforcers. Pharmacol Biochem Behav 17: 563–567.
Carroll ME, Gao Y, Brimijoin S, Anker JJ (2011). Effects of cocaine hydrolase on cocaine self-administration under a PR schedule and during extended access (escalation) in rats. Psychopharmacology (Berl) 213: 817–829.
Carroll ME, Zlebnik NE, Anker JJ, Kosten TR, Orson FM, Shen X et al (2012). Combined cocaine hydrolase gene transfer and anti-cocaine vaccine synergistically block cocaine-induced locomotion. PLoS One 7: e43536.
Crumb WJ Jr., Clarkson CW (1992). Characterization of the sodium channel blocking properties of the major metabolites of cocaine in single cardiac myocytes. J Pharmacol Exp Ther 261: 910–917.
Gao Y, Atanasova E, Sui N, Pancook JD, Watkins JD, Brimijoin S (2005). Gene transfer of cocaine hydrolase suppresses cardiovascular responses to cocaine in rats. Mol Pharmacol 67: 204–211.
Gao Y, Brimijoin S (2004). An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther 310 1046–1052.
Gao Y, Brimijoin S (2006). Viral transduction of cocaine hydrolase in brain reward centers. Cell Mol Neurobiol 26: 357–363.
Gao Y, Orson FM, Kinsey B, Kosten T, Brimijoin S (2010). The concept of pharmacologic interception as a treatment for drug abuse. Chem Biol Interact 187: 421–424.
Geng L, Gao Y, Chen X, Hou S, Zhan CG, Radic Z et al (2013). Gene transfer of mutant mouse cholinesterase provides high lifetime expression and reduced cocaine responses with no evident toxicity. PLoS One 8: e67446.
Grubic Z, Sket D, Brzin M (1988). Iso-OMPA-induced potentiation of soman toxicity in rat correlates with the inhibition of plasma carboxylesterases. Arch Toxicol 62: 398–399.
Inaba T (1989). Cocaine: pharmacokinetics and biotransformation in man. Can J Physiol Pharmacol 67: 1154–1157.
Johanson CE, Fischman MW (1989). The pharmacology of cocaine related to its abuse. Pharmacol Rev 41: 3–52.
Kalow W (2004). Human pharmacogenomics: the development of a science. Hum Genomics 1: 375–380.
Mesulam M (2003). Butyrylcholinesterase in the normal and Alzheimer brain. In: Giacobini E (ed) Butyrylcholinesterase, Its Function and Inhibitors. Martin Dunitz: New York. pp 29–37.
Murthy V, Gao Y, Geng L, Lebrasseur N, White T, Brimijoin S (2013). Preclinical studies on neurobehavioral and neuromuscular effects of cocaine hydrolase gene therapy in mice. J Mol Neurosci, (2013). PMID: 24085526 (doi:10.1007/s12031-013-0130-5).
National Research Council (2011) Guide for the Care and Use of Animals 8th edn The National Academies Press: Washington, DC.
Pan Y, Gao D, Yang W, Cho H, Yang G, Tai HH et al (2005). Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci USA 102: 16656–16661.
Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL (1996). A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA 93: 13565–13570.
Seiden LS, Sabol KE, Ricaurte GA (1993). Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol 33: 639–677.
Soliday FK, Conley YP, Henker R (2010). Pseudocholinesterase deficiency: a comprehensive review of genetic, acquired, and drug influences. Aana J 78: 313–320.
Sun H, Pang YP, Lockridge O, Brimijoin S (2002). Re-engineering butyrylcholinesterase as a cocaine hydrolase. Mol Pharmacol 62: 220–224.
Tucker GT, Lennard MS, Ellis SW, Woods HF, Cho AK, Lin LY et al (1994). The demethylenation of methylenedioxymethamphetamine (‘ecstasy’) by debrisoquine hydroxylase (CYP2D6). Biochem Pharmacol 47: 1151–1156.
Weber A, Butterweck H, Mais-Paul U, Teschner W, Lei L, Muchitsch EM et al (2011). Biochemical, molecular and preclinical characterization of a double-virus-reduced human butyrylcholinesterase preparation designed for clinical use. Vox Sang 100: 285–297.
Xue L, Hou S, Yang W, Fang L, Zheng F, Zhan CG (2013). Catalytic activities of a cocaine hydrolase engineered from human butyrylcholinesterase against (+)- and (−)-cocaine. Chem Biol Interact 203: 57–62.
Zheng F, Yang W, Ko MC, Liu J, Cho H, Gao D et al (2008). Most efficient cocaine hydrolase designed by virtual screening of transition states. J Am Chem Soc 130: 12148–12155.
Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME (2010). Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology 209: 113–125.
Acknowledgements
We thank Cole Batty, James Brown, Luke Bushman, Clare Chamberlain, Seth Johnson, Sarah Korthauer, Torie Lepak, Nathan Omdalen, Heather Veglahn, and Ashley Xiong for technical assistance and Krista Walkowiak, DVM, for veterinary care. Funding for this study was provided by the National Institute on Drug Abuse (NIDA) grant DP1 DA031340 (SB); NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zlebnik, N., Brimijoin, S., Gao, Y. et al. Long-Term Reduction of Cocaine Self-Administration in Rats Treated with Adenoviral Vector-Delivered Cocaine Hydrolase: Evidence for Enzymatic Activity. Neuropsychopharmacol 39, 1538–1546 (2014). https://doi.org/10.1038/npp.2014.3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.3
Keywords
This article is cited by
-
Environmental, genetic and epigenetic contributions to cocaine addiction
Neuropsychopharmacology (2018)
-
Plant-expressed cocaine hydrolase variants of butyrylcholinesterase exhibit altered allosteric effects of cholinesterase activity and increased inhibitor sensitivity
Scientific Reports (2017)
-
Reaction pathway for cocaine hydrolase-catalyzed hydrolysis of (+)-cocaine
Theoretical Chemistry Accounts (2016)
-
Reward and Toxicity of Cocaine Metabolites Generated by Cocaine Hydrolase
Cellular and Molecular Neurobiology (2015)