Abstract
The mechanistic target of rapamycin complex 1 (mTORC1) is necessary for synaptic plasticity, as it is critically involved in the translation of synaptic transmission-related proteins, such as Ca2+/Calmodulin-dependent kinase II alpha (CAMKIIα) and AMPA receptor subunits (GluAs). Although recent studies have implicated mTORC1 signaling in drug-motivated behavior, the ineffectiveness of rapamycin, an mTORC1 inhibitor, in suppressing cocaine self-administration has raised questions regarding the specific role of mTORC1 in drug-related behaviors. Here, we examined mTORC1’s role in three drug-related behaviors: cocaine taking, withdrawal, and reinstatement of cocaine seeking, by measuring indices of mTORC1 activity and assessing the effect of intra-cerebroventricular rapamycin on these behaviors in rats. We found that withdrawal from cocaine self-administration increased indices of mTORC1 activity in the nucleus accumbens (NAC). Intra-cerebroventricular rapamycin attenuated progressive ratio (PR) break points and reduced phospho-p70 ribosomal S6 kinase, GluA1 AMPAR, and CAMKIIα levels in the NAC shell (NACsh) and core (NACc). In a subsequent study, we treated rats with intra-NACsh infusions of rapamycin (2.5 μg/side/day for 5 days) during cocaine self-administration and then tracked the expression of addiction-relevant behaviors through to withdrawal and extinction. Rapamycin reduced drug seeking in signaled non-drug-available periods, PR responding, and cue-induced reinstatement, with these effects linked to reduced mTORC1 activity, total CAMKIIα, and GluA1 AMPAR levels in the NACsh. Together, these data highlight a role for mTORC1 in the neural processes that control the expression and maintenance of drug reward, including protracted relapse vulnerability. These effects appear to involve a role for mTORC1 in the regulation of GluA1 AMPARs and CAMKIIα in the NACsh.
Similar content being viewed by others
INTRODUCTION
Dysregulated signaling through a number of neuronal surface receptors on nucleus accumbens (NAC) medium spiny neurons (MSNs), including dopamine, ionotropic, and metabotropic glutamate receptors, as well as neurotrophin receptors, has been linked with expression of addiction-relevant behaviors (Brami-Cherrier et al, 2002; Dayas et al, 2012; Russo et al, 2009). These receptor systems can signal through the MAPK-ERK and PI3K/AKT pathway to influence gene transcription and protein translation, with subsequent effects on neural activity and behaviors (McGinty et al, 2008; Russo et al, 2009). Interestingly, recent studies indicate that these receptor systems can recruit the mechanistic target of rapamycin (mTOR; Hoeffer and Klann 2010), a serine-threonine kinase that, together with Raptor and other interacting proteins, forms the mechanistic target of rapamycin complex 1 (mTORC1; Hoeffer and Klann 2010). mTORC1 is expressed in both neuronal cell bodies and neuronal dendrites and controls key steps in protein translation initiation via the phosphorylation and activation of translation initiation factors such as the p70 ribosomal S6 kinase (p70s6k) and eukaryotic translation initiation factor-4E-binding protein (Hoeffer and Klann 2010). It is now believed that mTORC1 plays a role in the synaptic plasticity processes of LTP (Cammalleri et al, 2003) and LTD (Mameli et al, 2007). The role of mTORC1 in these mechanisms is linked to the function of this complex in regulating the translation of specific synaptic proteins, such as Ca2+/Calmodulin-dependent kinase II alpha (CAMKIIα) and AMPAR receptor subunits (GluA1s; Schratt et al, 2004; Slipczuk et al, 2009).
The formation of mTORC1 can be blocked by rapamycin, an allosteric mTOR inhibitor that acts by displacing Raptor, inhibiting its capacity to phosphorylate mTOR substrates (Hoeffer and Klann, 2010). Studies using rapamycin have implicated mTORC1 in late phase (protein synthesis dependent) LTP and LTD (Cammalleri et al, 2003; Stoica et al, 2011), as well as associative learning processes, including inhibitory avoidance memory and fear conditioning (Blundell et al, 2008; Parsons et al, 2006). More recently, experiments using rapamycin have implicated mTORC1 signaling in addiction-relevant behaviors and in alcohol-related memory consolidation (Barak et al, 2013). Both acute systemic and intra-NAC injections of rapamycin attenuated alcohol-drinking behaviors and conditioned place preference (CPP) for alcohol (Neasta et al, 2010). Acute systemic rapamycin treatment also suppressed the expression of cocaine CPP, behavioral sensitization to cocaine (Bailey et al, 2012), and the sensitization of meth-amphetamine CPP (Narita et al, 2005). These findings indicate that mTORC1 may have a role in controlling the processes responsible for the strengthening of drug/cue associations. NAC mTORC1 inhibition also attenuated cue-induced reinstatement of cocaine seeking but, interestingly, had no effect on cocaine self-administration under a fixed-ratio 1 (FR1) schedule of reinforcement (Wang et al, 2010b).
In the present study, we investigated the role of mTORC1 in drug-related behaviors by first assessing the effects of cocaine withdrawal on indices of mTORC1 activity. Next, we determined the role of mTORC1 in drug seeking by examining the effects of central (intra-cerebroventricular; i.c.v.) mTORC1 inhibition on lever pressing for cocaine using a progressive ratio (PR) schedule of reinforcement, a procedure thought more likely to reveal the neural processes underlying drug-motivated behavior. In these experiments, we assessed changes in mTORC1-signaling pathway proteins in the NAC shell (NACsh) and core (NACc). Cocaine-motivated behaviors have been shown to require proteins that enhance glutamate signaling in NAC MSNs (Anderson et al, 2008; Conrad et al, 2008; Wolf, 2010), so we assessed GluA1 AMPAR subunit and CAMKIIα levels in these brain regions after rapamycin treatment. Importantly, given the potential for mTORC1 inhibition to prevent neuroadaptations that promote synaptic plasticity and memory (Barak et al, 2013; Lin et al, 2014), we also tested whether rapamycin delivered to the NACsh during the cocaine-taking phase might have protracted effects on the expression of addiction-relevant behaviors including relapse-like behavior. We then assessed changes in NAC mTORC1 signaling pathway and GluA1 and CAMKIIα proteins, as they have recently been found to also play a role in cocaine relapse (Anderson et al, 2008).
MATERIALS AND METHODS
General Methods
For details on intravenous and intracranial surgery, drug preparation and behavioral testing equipment, see Supplementary Material (S1).
Self-Administration Training
Rats were trained to respond on the right lever for a cocaine infusion (0.25 mg/0.1 ml i.v. delivered over 4 s; (James et al, 2011a, 2010)) on an FR1 schedule (3 h/day, 5 days/week). Infusions were followed by the illumination of a white cue light above the active lever signaling a 20-s timeout period. Rats were limited to earning a maximum of 20 infusions during these sessions to prevent overdosing. Once stable responding was established (±10% rewarded responses over three sessions), the 3-h daily sessions were divided into alternating ‘drug-available’ and ‘non-drug-available’ periods, as described elsewhere (Brown et al, 2011; Deroche-Gamonet et al, 2004; Kasanetz et al, 2010). Briefly, the 40-min drug-available periods were signaled by a constant 70 dB white noise, whereas the 20-min non-drug-available periods were signaled by constant illumination of the white house-light. Cocaine infusions were only possible during drug-available periods, whereas lever presses during non-drug-available periods were recorded but had no scheduled consequences. An FR1 schedule was employed during the initial drug-available sessions and was then increased to FR5 with unrestricted access to drug. During these sessions, responses on the left ‘inactive’ lever were recorded but did not result in a drug infusion. Following the FR5 discrimination training sessions, rats underwent intracranial surgery (see Supplementary Material S1). After 7 days recovery from surgery, rats underwent an additional 5 days of cocaine self-administration on the FR5 drug-available/non-drug-available program.
For saline control groups, rats were prepared with an intravenous catheter and received ‘yoked’ saline infusions over a similar period of time to rats trained to self-administer cocaine. The number and temporal pattern of saline infusions was calculated based on the same parameters averaged across cocaine self-administering rats.
PR Testing
PR testing was conducted under drug-available conditions using the schedule 5, 10, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, 268, 345, 445, 573, 737, 947, 1218, 1566, 2012, 2585 presses required for one infusion. PR testing was performed over 5 h, however, PR sessions were programmed to shut down if a lever press was not recorded within 45 min of a previous lever press.
Spontaneous Locomotor Activity Assay
Locomotor activity was assessed in cocaine-naive rats using previously published procedures (James et al, 2011b). Rapamycin or vehicle was administered 3 h before testing.
Extinction and Cue-Induced Reinstatement Testing
Lever pressing was extinguished in 2 h extinction sessions until responding reached ⩽6 active lever presses over three consecutive sessions. Discriminative stimuli were withheld, along with drug infusions. One day after the last extinction session, rats were tested for reinstatement of drug seeking for 1 h by re-exposing rats to the cues paired with drug availability during self-administration training. Lever presses during reinstatement tests did not result in cocaine infusions but did result in illumination of the white cue light above the active lever (20 s).
Experiment One
Effect of cocaine self-administration on mTORC1 activity in the NAC
A group of rats (n=6) were trained to self-administer cocaine for a period of approximately 5 weeks. A separate group of rats received yoked saline over the same period (n=6). Both groups of rats were killed 24 h after the final self-administration (or yoked) session, and brains were processed for markers of mTORC1 activity in the NAC.
Experiment Two
Effect of i.c.v. rapamycin on PR responding
Rats acquired stable self-administration behavior on an FR1 schedule before being tested on a PR schedule. On days 1–3 of PR testing, baseline PR scores were obtained. On days 4–6, rats received an infusion of rapamycin (12.5 μg/μl, i.c.v., 1 μl/min for 2 min; n=10) or vehicle (n=9) 3 h before PR testing through an injector (28 gauge; Plastics One, VA, USA), which protruded 1 mm below the guide cannulae. The timing and dose of rapamycin was based on previous studies (Cota et al, 2008; Neasta et al, 2010). The injector remained in place for 60 seconds to ensure diffusion of the injectate. Rats were killed 24 h after the final PR session and brains were processed for markers of mTORC1 activity.
The effect of i.c.v. rapamycin on locomotor activity was assessed in a separate cohort of cocaine-naive rats (Supplementary Material S1). Rats from this group were killed 24 h after the final locomotor test and brains were assessed for markers of mTORC1 activity.
Experiment Three
Effect of intra-NACsh-directed rapamycin infusions on addiction-relevant behaviors
Rats were trained to discriminate between periods of signaled drug availability and non-drug availability, and then underwent cranial surgeries, as described in Supplementary Material S1. To facilitate intra-NACsh infusions, rats were gently restrained, stylets were removed and an injector (20.2 mm; 28 gauge; Plastics One) was inserted into each of the two guide cannulae. Rapamycin (n=17) or vehicle (n=17) was infused at a volume of 2.5 μg/0.5 μl/side over 1 min. For both cannuale, the injector remained in place for an additional 60 s to ensure diffusion of the injectate, after which stylets were replaced. Infusions took place once per day for 5 consecutive days. As rapamycin may interfere with memory consolidation processes when administered before learning (Parsons et al, 2006), approximately half of the rats (n=8) received rapamycin or vehicle 3 h before testing, whereas the remaining half (n=9) received treatment immediately following testing (immediately after the 3 h cocaine self-administration session). Importantly, no differences were observed between these two treatment regimes across all parameters measured (non-drug available responding (F1,14=0.03, P=0.98); PR responding: (F1,14=7.12, P=0.32); cue-induced reinstatement: (F1,9=0.60, P=0.97), data not shown), and as such, data were collapsed across both rapamycin treatment groups.
To assess the effects of intra-NACsh rapamycin treatment on addiction-like behavior, rats were assessed on three well-established behavioral indices of addiction vulnerability (Brown et al, 2011; Deroche-Gamonet et al, 2004; Kasanetz et al, 2010; for timeline, see Figure 4). First, across the 5 days of rapamycin treatment, rats were assessed for active lever responding during signaled periods of non-drug-availability. Second, on the three days following the last treatment session, rats were tested for motivation to obtain drug on a PR schedule identical to Experiment 2. Finally, in order to assess the long-term effects of rapamycin, we assessed reinstatement behavior after extinction training by re-exposing rats to cues paired with drug availability during self-administration training. Rats were killed 24 h after reinstatement testing and brains were processed for protein analysis.
We also sought to assess the effect of intra-NACsh treatment on the activity of the mTORC1 pathway in rats with no history of cocaine self-administration but that had similar exposure to the operant environment. To do this, a separate group of rats (n=6) received yoked saline infusions. Rats were killed 3 weeks after the final saline yoking session, which equated to the average time between the final self-administration session and kill in the cocaine-exposed group. Brains from the saline-yoked group were processed for markers of mTORC1 activity in a similar manner to cocaine-exposed rats.
Details of animal kill, western-blot analysis, and statistical analysis are outlined in Supplementary Material S1.
RESULTS
Experiment One
Withdrawal from cocaine self-administration increased indices of mTORC1 activity
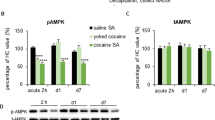
Rats trained to self-administer cocaine consumed on average a total of 542.59 (±24.86) mg/kg of cocaine over the training period. We compared the levels of total and phosphorylated mTOR protein in the NACc and NACsh of all treatment groups 24 h after the last saline or self-administered cocaine infusion. Cocaine self-administration increased both total mTOR (t(10)=−2.33, P=0.042) and phosphorylated mTOR levels (t(10)=−3.43, P=0.006) in the NACsh. In contrast, there was no effect of cocaine on total or phosphorylated mTOR levels in the NACc (P’s>0.05). Total p70s6k levels in the NACc were significantly elevated in cocaine self-administering rats compared with yoked saline controls (t(10)=−4.69, P<0.001). No differences were observed in phospho-p70s6k, GluA1, and CAMKIIα levels in either subregion (Figure 1).
Withdrawal from cocaine self-administration increased indices of mTORC1 activity. Compared with saline controls, rats with a history of cocaine self-administration demonstrated increased total-mTOR (a) and phospho-mTOR (b) in the NACsh but not NACc. Cocaine-exposed rats also demonstrated an increase in total-p70s6k in the NACc (c). No significant changes were observed in phospho-p70s6k (d), GluA1 (e) or CAMKIIα in the NAC (f). Data represent means±SEM. *P<0.05; ***P<0.001. n=6/group.
Experiment Two
Effect of mTORC1 inhibition on motivation to obtain cocaine under PR reinforcement conditions
A comparison of treatment groups revealed no differences in FR5 responding (P>0.05; Figure 2a) or the number of cocaine infusions before rapamycin treatment (P>0.05; data not shown). The effect of i.c.v. rapamycin on motivation to obtain cocaine was then assessed using PR tests over 3 consecutive days. Rapamycin-treated rats exhibited significantly lower break points than vehicle-treated controls on days 2 (F1,16=5.03, P=0.043) and 3 (F1,16=5.14, P=0.041) of PR testing (Figure 2b). Of note, i.c.v. rapamycin treatment was associated with a modest decrease in body weight over the treatment period (F1,16=4.65, P=0.047; Figure 2c).
Effect of mTORC1 inhibition on motivation to obtain cocaine under progressive ratio reinforcement conditions. Vehicle- or rapamycin-injected rats did not differ in terms of active or inactive lever responding in cocaine self-administration sessions before treatment (a). Rats that received i.c.v. rapamycin exhibited significantly lower break points on days 2 and 3 of progressive ratio testing as compared with controls (b). i.c.v. administration of rapamycin was associated with modest decreases in body weight on the final day of treatment (c). Data represent means±SEM. *P<0.05; ***P<0.001. n=9–10/group.
Effect of mTORC1 inhibition on CAMKIIα and GluA1 levels
In i.c.v.-treated rats, rapamycin significantly reduced total mTOR levels in the NACsh (t(10)=2.27, P=0.047) but not the NACc (P=0.13; Figure 3a). Interestingly, no differences were observed between treatment groups in terms of total p70s6k levels in either NAC subregion (P’s>0.05; Figure 3b), but significantly reduced phosphorylated p70s6k levels were observed in both the NACsh (t(10)=11.13, P<0.001) and NACc (t(10)=4.209, P=0.002) of rapamycin-treated rats (Figure 3c). We also assessed the effects of rapamycin treatment on the expression of synaptic proteins GluA1 and CAMKIIα. Rapamycin treatment reduced GluA1 levels in both the NACsh (t(10)=9.05, P<0.001) and NACc (t(10)=3.66, P=0.004; Figure 3d) and reduced total CAMKIIα levels in the NACsh (t(10)=4.76, P<0.001) and NACc (t(10)=2.58, P=0.03; Figure 3e).
Effect of i.c.v. mTORC1 inhibition on CAMKIIα and GluA1 levels. i.c.v. rapamycin administration significantly attenuated total mTOR levels in the NACsh but not NACc (a). Rapamycin had no effect on total p70s6k levels (b), but significantly reduced phospho-p70s6k levels in both the NACsh and NACc (c). Similarly, rapamycin treatment was associated with reduced GluA1 (d) and CAMKIIα (e) expression in NACsh and NACc. Data represent means±SEM. *P<0.05; **P<0.01; ***P<0.001. n=6/group.
For data outlining the effect of mTORC1 inhibition on CAMKIIα and GluA1 levels and locomotor activity in cocaine naive rats, see Supplementary Material S2.
Experiment Three
Effect of intra-NACsh mTORC1 inhibition on drug seeking in the signaled non-drug-available period and under PR conditions
Details of guide cannulae placement are described in Supplementary Material S3.
As with Experiment 2, rats were randomly allocated to treatment groups following self-administration training. Treatment groups did not differ in terms of overall cocaine self-administration (P=0.83, data not shown) or number of days taken to reach the treatment phase (P=0.57, data not shown). Importantly, rats from both groups were able to discriminate between drug-available and non-drug-available cues, as responding was significantly higher in drug-available sessions (F1,26=491.09, P<0.001). Over the treatment period, rapamycin-treated rats did not differ to controls in terms of lever responding during drug-available periods (P=0.58; Figure 4a) nor amount of cocaine consumed (P=0.84, data not shown). However, rapamycin-treated rats exhibited significantly fewer active lever responses in the non-drug-available periods as compared with controls across the treatment period (F1,26=6.89, P=0.014; Figure 4a). Throughout the treatment period, no differences were observed between treatment groups in terms of responding on the inactive lever (P>0.05; data not shown). In addition, rapamycin treatment had no effect on body weight over the treatment period (P>0.05, data not shown).
Effect of intra-NAC mTORC1 inhibition on drug-seeking and taking behaviors and progressive ratio responding. In both treatment groups, responding during periods of signaled drug availability was significantly higher than during periods of signaled non-drug availability. Treatment groups did not differ in their responding during periods of drug availability. However, intra-NACsh rapamycin treatment significantly reduced responding during periods of signaled non-drug-availability (a). In the week following treatment, rats that received intra-NACsh rapamycin exhibited significantly reduced break points (b). Treatment groups did not differ in terms of days taken to reach the extinction criterion (c). Rapamycin-treated rats exhibited significantly lower levels of cue-induced reinstatement of extinguished cocaine seeking, despite treatment occurring approximately 4 weeks prior (d). Data represent means±SEM. *P<0.05. n=14–15/group. DA, Drug-available; NDA, Non-drug-available; S+, Presentation of 'drug-available' discriminative stimulus.
One day following rapamycin treatment, rats were assessed for motivation to obtain cocaine on a PR schedule. Rapamycin-treated rats exhibited a significantly lower average break point than vehicle-treated controls across the 3 days of PR testing (t (27)=2.553, P=0.017; Figure 4b). A similar analysis revealed no effect of treatment on inactive lever responding (P>0.05, data not shown).
Effect of intra-NACsh mTORC1 inhibition on cue-induced reinstatement of drug seeking
Two rapamycin-treated and two vehicle-treated rats did not complete the entire experimental protocol, precluding collection of extinction and reinstatement data for these rats. All other rats took an average of 20 days to reach the extinction criterion, and this did not differ between treatment groups (F1,25=1.841, P=0.188; Figure 4c). All rats exhibited a significant reinstatement of responding on the active lever following presentation of the drug-available cue during reinstatement testing, as compared with extinction responding. Reinstatement on the active lever was significantly attenuated in rapamycin-treated rats, as compared with vehicle-treated controls (F1,25=5.929, P=0.023; Figure 4d). No differences were observed in terms of responding on the inactive lever during reinstatement testing (P=0.544; data not shown).
Effect of intra-NACsh mTOR inhibition on mTORC1 signaling and GluA1 and CAMKIIα levels after reinstatement
Intra-NACsh rapamycin-treated rats displayed no changes in total mTOR or p70s6k levels in either NAC subregion (P’s>0.05; Figure 5a and b). Importantly, however, in rapamycin-treated rats, significantly reduced levels of phosphorylated p70s6k within the NACsh were observed (t(10)=3.34, P=0.008; Figure 5c). Rapamycin treatment was also associated with significantly attenuated total GluA1 (t(10)=2.91, P=0.016) and total CAMKIIα (t(10)=2.66, P=0.024) levels in the NACsh, but had no effect on these parameters in the NACc (P’s>0.05; Figure 5d and e).
Effect of intra-NACsh mTORC1 inhibition on cue-induced reinstatement and CAMKIIα and GluA1 levels. Intra-NACsh vehicle- or rapamycin-treated rats did not differ in terms of total mTOR levels in the NAC (a). Similarly, total p70s6k levels did not differ between groups (b), but rapamycin treatment resulted in reduced phospho-p70s6k in the NACsh (c). Interestingly, rapamycin treatment resulted in reduced total GluA1 (d) and CAMKIIα (e) levels in the NACsh, but had no effect on these parameters in the NACc. Data represent means±SEM. *P<0.05; **P<0.01. n=6/group.
For data outlining the effect of mTORC1 inhibition on CAMKIIα and GluA1 levels and locomotor activity in cocaine naive rats, see Supplementary Material S4.
DISCUSSION
In the present study, we found evidence of increased mTORC1 activity in the NAC after withdrawal from cocaine self-administration. Further, we show that mTORC1 inhibition using rapamycin reduced the motivation to lever press for cocaine under PR conditions, with these effects being linked to a reduction in GluA1 AMPARs and CAMKIIα in the NAC. Importantly, in a separate experiment where we tracked the effect of mTORC1 inhibition on the expression of addiction-relevant behaviors over time, we found that intra-NACsh rapamycin did not alter cocaine self-administration under FR5 conditions. In contrast, in the week following treatment, intra-NACsh rapamycin reduced drug seeking during periods of signaled non-drug-availability and attenuated motivation to lever press for cocaine on a PR schedule. Most notably, a 40% reduction in cue-induced reinstatement of drug seeking was observed in rapamycin-treated rats, despite the fact that treatment ceased up to 4 weeks before testing. The protracted rapamycin-induced reductions in drug-seeking behavior were accompanied by biochemical evidence of reduced mTORC1 activity and reduced total GluA1 and CAMKIIα levels. These data suggest that mTORC1 reduces the translation of synaptic plasticity proteins in the NAC, an effect that ‘protects’ against the expression of drug-seeking behavior.
Effect of Cocaine Withdrawal on Indices of mTORC1 Activity
Based on changes in the levels of phospho-mTOR, our study indicates that after 24 h of cocaine withdrawal, mTORC1 activity is increased in the NACsh. These findings are highly consistent with the report of Neasta et al (2010), who showed that markers of mTORC1 activity were increased after withdrawal from alcohol. It is possible that changes in mTORC1 activity manifest on a different time scale in the NACsh versus NACc. Further work will be required to understand the significance of these findings given the different roles ascribed to NAC core in shell in drug-motivated behavior (Ito et al, 2004).
Intra-cerebroventricular mTORC1 Inhibition Reduces PR Responding and GluA1 and CAMKIIα Levels
In accordance with findings of Lu and colleagues (Wang et al, 2010b), we found that cocaine self-administration (FR5) was unaffected by intra-NAC rapamycin injections (Experiment 3). However, i.c.v. rapamycin reduced lever pressing for cocaine when PR conditions were required to obtain cocaine infusions. To date, data supporting the role for mTORC1 in psychostimulant reinforcement has been limited to CPP and sensitization studies (Bailey et al, 2012; Narita et al, 2005). However, mTORC1 inhibition has been reported to reduce low-effort alcohol drinking in mice and FR1 alcohol self-administration in rats (Neasta et al, 2010). With respect to biochemical changes induced by i.c.v. rapamycin, we found significant reductions in indices of mTORC1 activity and GluA1 AMPARs and CAMKIIα within the NAC. The magnitude of these effects was similar to previous studies (Barak et al, 2013; Neasta et al, 2010). These findings are interesting given that dopamine D1-like agonists increase AMPAR insertion into NAC MSNs through a process involving CAMKIIα (Anderson et al, 2008; Sun et al, 2008) and that both dopamine and CAMKIIα have been shown to be important for the expression of psychostimulant sensitization and drug-seeking behavior (Licata and Pierce, 2003; Loweth et al, 2008; Pierce et al, 1998). In fact, lentiviral-mediated knockdown of CAMKIIα in the NACsh reduced motivation to self-administer cocaine on a PR schedule (Wang et al, 2010a). Collectively, these data support a hypothesis in which mTORC1 acts as a key effector in the molecular pathway controlling the dynamic regulation of synaptic proteins within the NAC that are required for the expression and maintenance of drug reward. However, we cannot rule out effects of mTORC1 inhibition after i.c.v. rapamycin in other parts of the reward circuit, including the VTA (Mameli et al, 2007).
Inhibition of mTORC1 in the NACsh Reduces Drug-Seeking Behavior Including Reinstatement
We found that mTORC1 inhibition in the NACsh significantly reduced lever-pressing behavior during periods of signaled non-drug-availability—a putative measure of compulsive drug seeking. Further, in the week following rapamycin, treated rats demonstrated reduced PR break points. These findings are in line with our i.c.v. rapamycin data and support a role for mTORC1 in the neural processes that are invoked to elevate motivational performance. Importantly, after extinction training, rapamycin-treated rats demonstrated close to a 40% reduction in reinstatement of cocaine seeking. Notably, the time to reach the extinction criterion did not differ between vehicle and treatment groups, indicating that there was no impairment in extinction learning produced by prior rapamycin injections.
The exact mechanism responsible for the reduction in reinstatement behavior exhibited after rapamycin treatment is unclear. However, our data showing reduced synaptic protein levels are interesting on several levels. Cocaine seeking has been shown to require an increase in translation, trafficking, and signaling through GluA1 AMPARs in the NAC, and CAMKIIα has a well-established role in this process (Anderson et al, 2008; Conrad et al, 2008; Ferrario et al, 2011; Lisman et al, 2012; Opazo et al, 2010). Given the functional link between these glutamate signaling molecules and reinstatement, the protective effects of intra-NACsh rapamycin on drug seeking might be mediated by a disruption in withdrawal-induced increases in synaptic GluA1s that increase the propensity for drug seeking (Anderson et al, 2008; Conrad et al, 2008; Mameli et al, 2007; Wolf, 2010). An important additional consideration is the possibility that rapamycin treatment disrupted associative memory processes during the drug-taking phase. For example, an important recent study identified a role for mTORC1 in alcohol-cue memory reconsolidation (Barak et al, 2013). Further, intra-amygdala or hippocampal rapamycin suppresses the consolidation and reconsolidation of fear memories (Blundell et al, 2008; Parsons et al, 2006) and mTORC1 is required for cocaine-induced plasticity in VTA dopamine neurons (Mameli et al, 2007).
Effect of mTORC1 Inhibition in Cocaine Naïve Rats
To assess the effects of rapamycin on mTORC1 signaling in cocaine naive rats, we performed biochemistry on NAC tissue in rats that received i.c.v. or intra-NAC rapamycin at equivalent time points to cocaine-trained rats. Although subtle differences in mTORC1 signaling were observed between i.c.v.- and intra-NAC rapamycin-treated cocaine naive controls, in general these experiments revealed that rapamycin treatment produced a generalized perturbation in mTORC1 activity and consequently GluA1 and CAMKIIα levels.
A particularly interesting observation in the present study was the effect of rapamycin on mTORC1 activity seen more than 1 month after treatment. These effects were more pronounced in cocaine-treated rats. The apparent long-term effects of rapamycin on mTORC1 activity might involve rapamycin-induced reductions in mTOR complex 2 and consequently AKT activity or other compensatory changes in this signaling pathway produced by sub-chronic rapamycin (Huang et al, 2013). Indeed, acute but not chronic rapamycin is known to reduce mTOR complex 2-induced phosphorylation of AKT at Ser473 (Huang et al, 2013). However, because cocaine appears to upregulate mTORC1 activity, a more likely explanation for the reductions in mTORC1 activity and addition-relevant behaviors after rapamycin treatment is a restoration of normal/baseline function in this signaling pathway.
Notably, rapamycin treatment did not affect locomotor activity or produce other non-specific effects on baseline motivational performance (ie, FR5 cocaine responding), findings that support recent demonstrations that rapamycin does not affect responding for a natural rewards (Neasta et al, 2010; Wang et al, 2010b). Thus, reductions in baseline mTORC1 in drug naive rats may be insufficient to alter general motivation status.
CONCLUSIONS
The present study extends our understanding of the role of mTORC1 in the neural processes that control the expression and maintenance of drug reward, including protracted vulnerability to reinstatement. These findings are significant given the recent demonstrations that mTORC1 effectively reduces alcohol-motivated behaviors in rats (Barak et al, 2013; Neasta et al, 2010) and that acute systemic rapamycin suppressed cue-induced drug craving in abstinent heroin addicts (Shi et al, 2009). Although it is important to note the need to directly test the hypothesis that the rapamycin-induced reductions in mTORC1 activity and glutamate signaling molecules suppressed cocaine-seeking behavior, the present study indicates that mTORC1 inhibition can have long-lasting protective effects against drug-seeking behavior.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE et al (2008). CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci 11: 344–353.
Bailey J, Ma D, Szumlinski KK (2012). Rapamycin attenuates the expression of cocaine-induced place preference and behavioral sensitization. Addict Biol 17: 248–258.
Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V et al (2013). Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci 16: 1111–1117.
Blundell J, Kouser M, Powell CM (2008). Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem 90: 28–35.
Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J (2002). Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci 22: 8911–8921.
Brown AL, Flynn JR, Smith DW, Dayas CV (2011). Down-regulated striatal gene expression for synaptic plasticity-associated proteins in addiction and relapse vulnerable animals. Int J Neuropsychopharmacol 14: 1099–1110.
Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W et al (2003). Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci USA 100: 14368–14373.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y et al (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454: 118–121.
Cota D, Matter EK, Woods SC, Seeley RJ (2008). The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci 28: 7202–7208.
Dayas CV, Smith DW, Dunkley PR (2012). An emerging role for the Mammalian target of rapamycin in ‘pathological’ protein translation: relevance to cocaine addiction. Front Pharmacol 3: 13.
Deroche-Gamonet V, Belin D, Piazza PV (2004). Evidence for addiction-like behavior in the rat. Science 305: 1014–1017.
Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ et al (2011). Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)(+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology 61: 1141–1151.
Hoeffer CA, Klann E (2010). mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33: 67–75.
Huang W, Zhu PJ, Zhang S, Zhou H, Stoica L, Galiano M et al (2013). mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci 16: 441–448.
Ito R, Robbins TW, Everitt BJ (2004). Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci 7: 389–397.
James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV (2011a). Propensity to 'relapse' following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience 199: 235–242.
James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR et al (2010). Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One 5: e12980.
James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW et al (2011b). Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol 14: 684–690.
Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O et al (2010). Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science 328: 1709–1712.
Licata SC, Pierce RC (2003). The roles of calcium/calmodulin-dependent and Ras/mitogen-activated protein kinases in the development of psychostimulant-induced behavioral sensitization. J Neurochem 85: 14–22.
Lin J, Liu L, Wen Q, Zheng C, Gao Y, Peng S et al (2014). Rapamycin prevents drug seeking via disrupting reconsolidation of reward memory in rats. Int J Neuropsychopharmacol 17: 127–136.
Lisman J, Yasuda R, Raghavachari S (2012). Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci 13: 169–182.
Loweth JA, Baker LK, Guptaa T, Guillory AM, Vezina P (2008). Inhibition of CaMKII in the nucleus accumbens shell decreases enhanced amphetamine intake in sensitized rats. Neurosci Lett 444: 157–160.
Mameli M, Balland B, Lujan R, Luscher C (2007). Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317: 530–533.
McGinty JF, Shi XD, Schwendt M, Saylor A, Toda S (2008). Regulation of psychostimulant-induced signaling and gene expression in the striatum. J Neurochem 104: 1440–1449.
Narita M, Akai H, Kita T, Nagumo Y, Narita M, Sunagawa N et al (2005). Involvement of mitogen-stimulated p70-S6 kinase in the development of sensitization to the methamphetamine-induced rewarding effect in rats. Neuroscience 132: 553–560.
Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D (2010). Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci USA 107: 20093–20098.
Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P et al (2010). CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67: 239–252.
Parsons RG, Gafford GM, Helmstetter FJ (2006). Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci 26: 12977–12983.
Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW (1998). Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther 286: 1171–1176.
Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ (2009). Neurotrophic factors and structural plasticity in addiction. Neuropharmacology 56 (Suppl 1)): 73–82.
Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME (2004). BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci 24: 7366–7377.
Shi J, Jun W, Zhao LY, Xue YX, Zhang XY, Kosten TR et al (2009). Effect of rapamycin on cue-induced drug craving in abstinent heroin addicts. Eur J Pharmacol 615: 108–112.
Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH (2009). BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One 4: e6007.
Stoica L, Zhu PJ, Huang W, Zhou H, Kozma SC, Costa-Mattioli M (2011). Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci USA 108: 3791–3796.
Sun X, Milovanovic M, Zhao Y, Wolf ME (2008). Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci 28: 4216–4230.
Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J et al (2010a). Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology 35: 913–928.
Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF et al (2010b). Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci 30: 12632–12641.
Wolf ME (2010). The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci 33: 391–398.
Acknowledgements
We acknowledge the assistance of Mr Cameron Adams and Mr Jonathan Cvetanoski for their technical assistance with the behavioral component of the i.c.v. experiment. We also thank Mrs Helen Carpenter and Mrs Michelle McConachy for their technical assistance with the western-blot analyses. These studies were supported by funding from the Australian National Health and Medical Research Council, the Hunter Medical Research Institute, and the University of Newcastle through project grants to C.V.D.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
James, M., Quinn, R., Ong, L. et al. mTORC1 Inhibition in the Nucleus Accumbens ‘Protects’ Against the Expression of Drug Seeking and ‘Relapse’ and Is Associated with Reductions in GluA1 AMPAR and CAMKIIα Levels. Neuropsychopharmacol 39, 1694–1702 (2014). https://doi.org/10.1038/npp.2014.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.16
Keywords
This article is cited by
-
A functional eEF2K-eEF2 pathway in the NAc is critical for the expression of cocaine-induced psychomotor sensitisation and conditioned place preference
Translational Psychiatry (2022)
-
Ribosomal DNA transcription is increased in the left nucleus accumbens of heroin-dependent males
European Archives of Psychiatry and Clinical Neuroscience (2022)
-
VTA mTOR Signaling Regulates Dopamine Dynamics, Cocaine-Induced Synaptic Alterations, and Reward
Neuropsychopharmacology (2018)
-
Distinct miRNA expression in dorsal striatal subregions is associated with risk for addiction in rats
Translational Psychiatry (2015)