Abstract
Few individuals seeking treatment for marijuana use achieve sustained abstinence. The cannabinoid receptor agonist, Δ9-tetrahydrocannabinol (THC; dronabinol), decreases marijuana withdrawal symptoms, yet does not decrease marijuana use in the laboratory or clinic. Dronabinol has poor bioavailability, which may contribute to its poor efficacy. The FDA-approved synthetic analog of THC, nabilone, has higher bioavailability and clearer dose-linearity than dronabinol. This study tested whether nabilone administration would decrease marijuana withdrawal symptoms and a laboratory measure of marijuana relapse relative to placebo. Daily, nontreatment-seeking marijuana smokers (8 men and 3 women), who reported smoking 8.3±3.1 marijuana cigarettes/day completed this within-subject study comprising three, 8-day inpatient phases; each phase tested a different nabilone dose (0, 6, 8 mg/day, administered in counter-balanced order on days 2–8). On the first inpatient day, participants took placebo capsules and smoked active marijuana (5.6% THC) at six timepoints. For the next 3 days, they had the opportunity to self-administer placebo marijuana (0.0% THC; withdrawal), followed by 4 days in which active marijuana was available for self-administration (5.6% THC; relapse). Both nabilone dose conditions decreased marijuana relapse and reversed withdrawal-related irritability and disruptions in sleep and food intake (p<0.05). Nabilone (8 mg/day) modestly worsened psychomotor task performance. Neither dose condition increased ratings of capsule ‘liking’ or desire to take the capsules relative to placebo. Thus, nabilone maintenance produced a robust attenuation of marijuana withdrawal symptoms and a laboratory measure of relapse even with once per day dosing. These data support testing of nabilone for patients seeking marijuana treatment.
Similar content being viewed by others
INTRODUCTION
Marijuana use has increased in the United States, with an estimated 29.7 million residents smoking marijuana in 2011 compared with 25.9 million in 2008 (SAMHSA, 2012). Among those who try marijuana, 8–9% transition to dependence (Lopez-Quintero et al, 2011; Wagner and Anthony, 2002). Given the ubiquity of marijuana use (Compton et al, 2007; Johnston et al, 2010), this rate of transition results in a large absolute number of dependent individuals, a subset of whom eventually seeks treatment for their marijuana use (Hall, 2006; EMCDDA, 2009). Yet, the vast majority of patients in marijuana treatment relapse; few are able to achieve sustained abstinence (Stephens et al, 2000; Copeland et al, 2001; MTPRG, 2004; Budney et al, 2006; Kadden et al, 2007; Carroll et al, 2012).
One factor influencing marijuana relapse is a withdrawal syndrome (Haney et al, 2012), characterized by a time-dependent increase in irritability and anxiety, sleep disruption, and decreased food intake (Haney et al, 1999; Hart et al, 2002, Schierenbeck et al, 2008; Allsop et al, 2012). Oral, synthetic Δ9-tetrahydrocannabinol (THC; dronabinol; Marinol) administration (30–90 mg/day) under double-blind conditions selectively reduced withdrawal symptoms in both laboratory (Haney et al, 2004; Budney et al, 2007) and clinical (Levin et al, 2010) settings. These findings both demonstrate the pharmacological specificity of the withdrawal syndrome and suggest that dronabinol has potential utility for the treatment of marijuana dependence. Yet dronabinol (40–60 mg/day) has not been shown to decrease marijuana use, either in the laboratory (Haney et al, 2008) or in the clinic (Levin et al, 2010).
Clearly, the sine qua non of an effective treatment medication is decreased drug-taking, and dronabinol failed to decrease marijuana use. However, given that dronabinol attenuated marijuana withdrawal symptoms, and given the success of agonist-based therapies for the treatment of opioid (methadone, buprenorphine) and tobacco (nicotine replacement) dependence, these negative findings do not justify a blanket rejection of agonist replacement therapy for marijuana treatment. Cannabinoid agonists with potentially better medication profiles than dronabinol appear worthy of investigation.
Nabilone (Cesamet) is a potent, FDA-approved synthetic analog of THC that is well absorbed when administered orally and has better efficacy (Matsuda et al, 1990) and bioavailability (⩾60%) than dronabinol (⩽20%; Glass et al, 1979; Lemberger et al, 1982; McGilveray, 2005; Ben Amar, 2006). Drug-discrimination studies in marijuana smokers show that nabilone (3–5 mg) substituted for dronabinol (25–30 mg), and also shifted the discriminative-stimulus effects of dronabinol leftward (Lile et al, 2010, 2011). We conducted a recent study to directly compare the acute behavioral and physiological effects of nabilone (2, 4, 6, 8 mg) to dronabinol (10, 20 mg) in marijuana smokers, and found that both medications increased positive mood and were well tolerated (Bedi et al, 2012). Yet compared with dronabinol, nabilone had more dose-related effects, a slower onset of peak subjective effects (180–240 vs 90 min) and a longer duration of action (>6 vs 4 h), consistent with earlier studies (Glass et al, 1981; Lile et al, 2010, 2011). A long duration of action is a positive clinical feature in that less frequent dosing could increase medication compliance. An additional positive clinical feature is that unlike dronabinol, nabilone produces urinary metabolites distinct from those of marijuana (Fraser and Meatherall, 1989), allowing clinicians to distinguish medication compliance from ongoing marijuana use.
The objective of this placebo-controlled study was to determine if repeated nabilone administration selectively decreased symptoms of marijuana withdrawal and decreased a laboratory measure of marijuana relapse. Using an inpatient model, daily marijuana smokers, explicitly not seeking marijuana treatment, underwent several days of abstinence and then had the opportunity to ‘relapse,’ defined as marijuana self-administration at a financial cost, after a period of abstinence (Haney et al, 2008, 2010, 2012; Haney, 2009; Cooper et al, 2012). We assessed the effects of two nabilone doses relative to placebo across a range of outcomes: mood, psychomotor task performance, food intake, sleep, blood pressure, and tobacco cigarette smoking. In this way, the specificity by which nabilone influenced marijuana withdrawal and relapse could be defined within the context of other effects, such as, sedation, intoxication, psychomotor impairment, and orthostatic hypotension.
MATERIALS AND METHODS
Participants
Healthy marijuana smokers were solicited through advertisements in New York, NY, and were enrolled from March to July 2011. Inclusion criteria included: 18–50 years of age and current marijuana use (minimum three marijuana cigarettes/day, 5 days/week). No participant could: (1) be dependent on any other substance except tobacco cigarettes, (2) meet DSM-IV criteria for a current axis I disorder requiring medical intervention, (3) be taking medication, or (4) be seeking treatment for marijuana smoking. Assessments of health included physical examination, psychiatric evaluation, electrocardiogram, urinalysis, and blood chemistry panels. All participants signed a consent form approved by the New York State Psychiatric Institute (NYSPI) Institutional Review Board, which described the study, outlined possible risks, indicated that two different strength marijuana cigarettes would be tested, and stated that participants could receive an FDA-approved medication used to treat nausea. Volunteers were compensated for participation.
Procedures
Participants, in groups of three or four, lived in a residential laboratory in NYSPI. The laboratory has four private rooms, a recreational area, two bathrooms, and two vestibules. Output from a video- and audio-monitoring system terminating in a control room allows for continuous observation of participants (see Haney et al, 1999). The study comprised three, 8-day inpatient phases, with each phase testing a different dose of medication. Each inpatient phase was separated by at least 7 outpatient days for medication washout.
Before study onset, participants completed two, 3- to 4-h training sessions. Before each inpatient stay, participants completed two ‘sample’ sessions. In one, they smoked an active marijuana cigarette (5.6% THC, labeled ‘dose A’), and in the other, they smoked a placebo marijuana cigarette (0.0% THC, labeled ‘dose B’) using procedures described below. They were told that the strength of dose A and dose B would not change throughout the study, and that they should attend to how each dose made them feel as they would later make decisions about purchasing individual puffs of dose A and dose B.
Once inpatient, participants completed a sleep scale and a mood scale on awakening at 0815 hours. Between 0915 and 1645 hours, they completed six 30-min task and subjective-effects batteries. The recreation area was available at lunchtime and from 1700 to 2200 hours. At 2330 hours, participants completed a final mood scale and were given $50 in faux money representing a portion of their daily earnings. They were told that this money could be used to purchase individual marijuana puffs on self-administration days or exchanged for cash on study completion. Lights were turned off by 0000 hours.
Marijuana Administration
Participants each received a single marijuana cigarette (provided by NIDA) at each smoking occasion. Marijuana was administered using a cued-smoking procedure, where inhalation duration, time spent holding smoke in the lungs, and inter-puff interval was timed (Foltin et al, 1987). Each day, active or placebo marijuana was either experimenter-administered at no cost or was available to purchase for self-administration; participants were informed of that day’s condition at 0950 hours each morning.
During experimenter-administered days (first day of each inpatient phase), participants smoked three puffs of dose A (5.6% THC) at 1000, 1130, 1300, 1430, 1600, and 2200 hours. The purpose of this day was to standardize marijuana exposure before abstinence. On the subsequent 3 days, dose B (0.0% THC) was available for self-administration at these six timepoints (withdrawal), followed by 4 days when dose A was available for self-administration (relapse). Participants could purchase up to three puffs of the available dose at each timepoint. The cost was $9 for the first puff of the day, and $2 for all subsequent puffs. Participants smoked self-administered marijuana in private so that others were blind to their choice. Money not spent on marijuana was received at study conclusion
Capsule Administration
The NYSPI Research Pharmacy packaged medication in size 00 opaque capsules. Medication administration was double-blind and counter-balanced across participants. As our objective was to assess nabilone’s effects on marijuana withdrawal and relapse, only placebo capsules were administered on the first inpatient day of each phase, when marijuana was experimenter-administered. The effects of three nabilone conditions on marijuana withdrawal and relapse were assessed: 0, 6, and 8 mg/day. Dosing occurred twice/day at 0900 and 1800 hours: 0 mg BID, 6 mg at 0900 and 0 mg at 1800 hours, and 4 mg BID (8 mg/day).
Our original dosing schedule, based on our dose-ranging study (Bedi et al, 2012), was 0, 4, and 6 mg BID. However, the first participant receiving 6 mg BID had the dose lowered to 6 mg once daily, based on the investigators’ assessment of over-intoxication (the participant completed all tasks but appeared sedated and repeatedly had heart rate <50 b.p.m. precluding dose administration). Thus, this individual received 3 rather than 7 days of 6 mg BID. We then modified the dose schedule from 6 mg BID to 6 mg/day for all subsequent participants.
As a possible side effect of nabilone is orthostatic hypotension (Glass et al, 1981), blood pressure was taken twice before each capsule administration: after participants had been seated for at least 1 min and then after they had been standing for 1 min. Capsules were not administered if: (1) seated SP was >160 mm Hg or SP decreased by ⩾20 mm Hg on standing, if (2) DP was ⩾110 mm Hg or decreased by >10 mm Hg on standing, or if HR was <50 or ⩾120 b.p.m.
Task Battery and Mood Scales
Each task battery consisted of a 3-min digit-symbol substitution task (DSST), a 3-min repeated-acquisition task (RAT), a 10-min divided attention task (DAT), a 10-min rapid information task (RIT), and an immediate and delayed digit-recall task. The battery measures aspects of learning, memory, vigilance, and psychomotor ability (Foltin et al, 1996).
A 44-item computerized subjective-effects questionnaire visual analog scale (VAS), comprising a series of 100-mm lines labeled ‘not at all’ (0 mm) at one end and ‘extremely’ at the other end, was completed 8 times per day. The VAS included mood, physical symptom, and drug effect descriptors; participants were instructed to rate the extent to which each descriptor applied to them at that moment. Based on a cluster analysis, we used arithmetic means of individual item scores to reduce 32 of the 44 items into six subscales: irritable (‘irritable,’ ‘miserable’); anxious (eg, ‘anxious,’ ‘restless’); bad effect (eg, ‘depressed,’ ‘upset stomach’); tired (eg, ‘tired,’ ‘sedated’); social (eg, ‘friendly,’ ‘talkative’); and high (‘high,’ ‘good effect’). We also analyzed VAS ratings of ‘hunger’ and drug craving: ‘I want…Marijuana,’ ‘Alcohol,’ and ‘Cigarettes.’ A Drug-Effect Questionnaire (Evans et al, 1995) was administered twice/day to assess ratings of capsule effects.
Food
Each morning, participants received a box of food containing a wide variety of meal items, snacks, and beverages. Frozen meals (n=20) and additional units of any item were available by request. Participants were instructed to scan custom-designed bar codes whenever they ate or drank, specifying substance and portion. Food was not available between 2330 and 0815 hours.
Sleep
Subjective ratings of the previous night’s sleep were obtained by having participants complete a seven-item VAS sleep questionnaire each morning (Haney et al, 2004). Objective measurement of sleep efficiency, defined as the percentage of time spent asleep during the lights-out period (0000–0800 hours), was obtained using the wrist-worn Actiwatch Activity Monitoring System (Respironics Company, Bend, OR).
Tobacco Cigarette Smoking
Participants were permitted to smoke cigarettes ad libitum. The number of tobacco cigarettes smoked was recorded by counting cigarette butts in each participant’s ashtray each evening.
Data Analysis
Repeated-measures analyses of variance with planned comparisons were used to determine the effect of each medication dose on marijuana withdrawal and relapse. Behavioral outcomes included: the number of marijuana puffs purchased during the relapse phase, daily peak subjective-effects ratings, drug craving, task performance, number of cigarettes smoked per day, objective and subjective sleep measures, food intake (total energy intake, percent macronutrient, number, and caloric content of individual eating occasions, defined as beginning with onset of food consumption and ending at the first pause in food reporting >10 min), body weight, seated and orthostatic blood pressure, and heart rate. There were two within-group factors: medication dose and inpatient day. One planned comparison assessed if there was a difference between active marijuana administration and marijuana abstinence (defined as mean peak values on the second and third day of abstinence). Planned comparisons tested if there was an effect of each nabilone dose compared with placebo on marijuana withdrawal and relapse, that is, marijuana self-administration on each day of active marijuana availability following an abstinence period. Results were considered statistically significant at p-values<0.05. Huynh–Feldt corrections were used, when appropriate. Note that a recent analysis of the factors predicting marijuana relapse in the laboratory included data from the placebo phase of this study (n=11) along with data (n=40) from the placebo phase of four other studies (Haney et al, 2012).
RESULTS
Participant Characteristics
Table 1 presents demographic data on the 11 marijuana smokers who completed the study. A twelfth participant enrolled but quit during a withdrawal phase. She reported being intensely irritated by another participant and feared she would respond aggressively. She was taking placebo medication at the time.
Marijuana Relapse
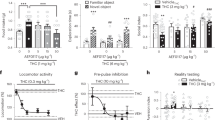
Figure 1 shows the mean number of marijuana puffs self-administered per day as a function of nabilone dose. On the first day, active marijuana was available, both the 6 mg (F(1, 120)=5.52) and the 8 mg (F(1, 120)=6.78) nabilone conditions significantly (p<0.05) decreased marijuana self-administration relative to placebo. On the subsequent 3 days that followed the initial opportunity to relapse, both nabilone doses continued to significantly (p<0.01) decrease marijuana self-administration relative to placebo (6 mg: F(1, 120)=21.66, 8 mg: F(1, 120)=10.87).
Average number of marijuana puffs self-administered on the first day of active marijuana availability (day 1) and on the subsequent three days of active marijuana availability (days 2–4) as a function of nabilone dose. Asterisks indicate a significant difference between each nabilone dose and placebo nabilone (*p<0.05; **p<0.01). Error bars represent ±SEM.
Marijuana Withdrawal
Subjective-effects ratings and drug craving
VAS ratings
Figure 2 and Table 2 portray outcome as a function of marijuana and nabilone dose condition. Note, degrees of freedom for all analyses are (1140) unless otherwise indicated. Under placebo nabilone conditions, marijuana abstinence was associated with significant (p<0.05) increases in cluster ratings of ‘irritable’ (F=10.42), ‘anxious’ (F=5.29), and ‘bad effect’ (F=14.48) and decreased ratings of ‘hungry’ (F=20.14), marijuana craving (F=7.10), and cluster ratings of ‘high’ (F=41.76) and ‘social’ (F=10.24). Craving for tobacco cigarettes also decreased by 20% during marijuana abstinence (F=8.41, p<0.02). Nabilone significantly (p<0.05) decreased ratings of ‘irritable’ (6 mg: F=19.38; 8 mg: F=16.75) and ‘bad effect’ (6 mg: F=6.20) compared with placebo, while conversely, high-dose nabilone (8 mg) further decreased ratings of ‘social’ (F=4.76) and marijuana craving (6 mg: F=7.65; 8 mg: F=4.60). Neither marijuana nor nabilone condition significantly affected cluster ratings of ‘tired’ (data not shown).
Mean effects on mood and sleep measures during marijuana administration (5.6%) and during marijuana abstinence as a function of nabilone dose. Maximum score for ratings=100 mm. Each graph represents data collected in 11 participants except for sleep efficiciency; because of equipment malfunction, this analysis included 10 rather than 11 participants. Asterisks indicate a significant difference between each nabilone dose and placebo medication (*p<0.05; **p<0.01). Number signs indicate a significant difference during marijuana administration and during marijuana abstinence under placebo medication conditions (#p<0.05; ##p<0.01). Error bars represent ± SEM.
Capsule ratings
Under placebo nabilone conditions, marijuana abstinence was associated with significant (p<0.05) changes in capsule ratings: participants rated that they ‘liked’ the capsules less (F=9.06), rated them as less ‘good’ (F=7.45), and were less willing to ‘take them again’ (F=19.19) compared with active marijuana administration (data not shown). Nabilone had no significant effect on capsule ratings relative to placebo.
Sleep
As shown in Figure 2, under placebo nabilone conditions, marijuana abstinence was associated with significantly (p<0.05) lower estimates of the number of hours slept (F=6.65), and lower ratings of ‘slept well’ (F=8.36), ‘clear-headed’ (F=4.92), and increased ratings of ‘woke often’ (F=14.60) compared with active marijuana administration. Nabilone reversed these effects. Compared with placebo capsules, nabilone significantly (p<0.05) increased estimated number of hours slept (6 mg: F=20.66; 8 mg: F=18.31), and increased ratings of ‘slept well’ (6 mg: F=6.92; 8 mg: F=9.94), while the highest nabilone dose (8 mg) significantly reduced ratings of ‘woke often’ (F=6.46).
Food intake and body weight
Figure 3 shows that under placebo nabilone conditions, marijuana abstinence was associated with a substantial decrease in total daily calories consumed (F=44.26, p<0.0001) compared with active marijuana administration. During abstinence, participants had fewer eating occasions (F=25.75, p<0.0001), and derived a significantly (p<0.05) lower percentage of total calories from fat (F=8.35) and a larger percentage of calories from carbohydrates (F=4.58) compared with marijuana administration (Table 2). Correspondingly, body weight fell significantly following marijuana abstinence relative to marijuana administration (Figure 3: F(1,180)=20.02, p<0.001).
Mean effects on food intake during marijuana administration (5.6%) and during marijuana abstinence as a function of nabilone dose. See Figure 2 for details.
Nabilone reversed the effects of marijuana abstinence on food intake. Both nabilone doses significantly increased caloric intake relative to placebo (6 mg: F=84.39; 8 mg: F=158.71, p<0.0001; Figure 3). This effect was due to both a significant (p<0.01) increase in the number of daily eating occasions (6 mg: F=10.90; 8 mg: F=10.27) and an increase in the caloric content of each eating occasion (6 mg: F=23.75; 8 mg: F=48.28). Further, nabilone altered the proportion of macronutrient intake, selectively increasing the percentage of calories derived from fat (6 mg: F=6.50; 8 mg: F=17.06) while reducing the percentage of calories derived from protein (6 mg: F=5.62; 8 mg: F=7.74) and carbohydrates (8 mg: F=7.55) compared with placebo capsules (Table 2).
Task performance
Under placebo nabilone conditions, marijuana abstinence resulted in significantly better performance on the DSST, with participants entering more patterns (F=11.13, p<0.01) compared with marijuana administration (Table 2). The high nabilone dose condition (8 mg) significantly (p<0.05) worsened task performance on a range of tasks as compared with placebo capsules: the number of patterns entered on the DSST (F=13.92) was reduced, and participants entered fewer sequences (F=7.76) on the RAT. On the DAT, nabilone (8 mg) decreased accuracy tracking the moving ball (F=26.1, p<0.0001) and increased latency to respond to a distracter symbol (F=15.5, p<0.01; data not shown) compared with placebo.
Blood pressure and heart rate
As shown in Table 2, under placebo nabilone conditions, marijuana abstinence was associated with small but significant (p<0.05) increases in seated SP (F=4.19), DP (F=6.74), and heart rate (F=6.16). Both doses of nabilone significantly (p<0.005) lowered seated SP (6 mg: F=12.02; 8 mg: F=10.08) and DP (6 mg: F=32.91; 8 mg: F=37.29) relative to placebo. Orthostatic blood pressure did not significantly vary as a function of nabilone or marijuana condition. However, of the 528 possible doses administered in the study, capsules were not given on four occasions because of orthostatic hypotension: one participant missed a placebo capsule administration because of a drop in SP on standing (>20 mm Hg). In the 8 mg nabilone condition, capsule administration (4 mg) was withheld for three participants on a single occasion: on standing, two had a decrease in DP (⩾10 mmHg) and one was normotensive but became dizzy.
Tobacco cigarette smoking
Neither marijuana abstinence nor nabilone condition significantly influenced the mean number of tobacco cigarettes smoked (8.8±5.6 cigarettes/day).
DISCUSSION
Nabilone significantly reversed characteristic symptoms of marijuana withdrawal, including worsened mood, disrupted sleep, and decreased food intake. Most critical for its potential clinical utility, both nabilone dose conditions also decreased a laboratory measure of marijuana relapse: participants chose to smoke fewer individual doses of marijuana while maintained on nabilone as compared with placebo. Nabilone significantly decreased relapse to active marijuana the first day it became available and persisted in decreasing marijuana self-administration on subsequent days of active marijuana availability, suggesting that nabilone not only lessens the likelihood of relapse occurring, but appears to lessen marijuana use even among those who have relapsed. This may have important clinical implications, as many patients in drug treatment eventually sample their abused drug after a period of abstinence, but a medication that prevents a return to pretreatment levels of drug use can have a significant impact on long-term outcome.
It is important to note that few medications, including dronabinol, have been shown to disrupt marijuana self-administration among abstinent, nontreatment-seeking, research volunteers, even at dronabinol doses large enough to produce intoxication (Haney et al, 2008). Medications, such as mirtazapine and quetiapine substantially reversed withdrawal-related disruptions in sleep and food intake, yet still did not decrease marijuana relapse in the laboratory (Haney et al, 2010; Cooper et al, 2012). Although the motivation driving relapse in this model presumably differs from that of a patient in treatment, medication effects on drug self-administration in the human laboratory have been shown to predict medication efficacy in the clinic (Haney and Spealman, 2008; Comer et al, 2008; Haney, 2009; Levin et al, 2010). Thus, the demonstration that nabilone decreased marijuana self-administration in the laboratory supports its clinical testing for the treatment of marijuana dependence.
Many of nabilone’s effects were likely related to the timing of its administration. The 6 mg dose was only administered once in the morning, while the 8 mg/day condition was administered in divided dose in the morning (4 mg: 0900 hours) and in the early evening (4 mg: 1800 hours). Although both dose conditions improved sleep, nabilone had more pervasive effects on sleep when administered in the evening nearer to bedtime. However, this dosing condition (8 mg/day) was also associated with modest decrements in psychomotor task performance and decreased ratings of sociability. Acute nabilone (6 and 8 mg) administration to non-abstinent marijuana smokers also produced modest decrements in task performance (Bedi et al, 2012). By contrast, lower nabilone doses (1–5 mg) produced few such effects in marijuana smokers (Lile et al, 2010, Bedi et al, 2012). Given that once per day dosing (6 mg) reduced both relapse and most symptoms of withdrawal, this regimen may be sufficient for improving marijuana treatment outcome without the potential cost of minor psychomotor slowing. It appears that nabilone’s slow onset of peak subjective effects and long duration of action, even relative to dronabinol, another slow-onset cannabinoid agonist (Kalliomäki et al, 2012; Bedi et al, 2012), may explain why this medication had robust effects even when given just once in the morning. Nabilone also appears to have active metabolites with longer half-lives than the parent compound, perhaps contributing to its long-lasting effects (eg, Rubin et al, 1977).
The 8 mg/day nabilone condition was also more likely to produce lowered blood pressure or dizziness, exceeding our criterion for capsule administration, although <1% of possible doses were skipped. Marijuana withdrawal alone produced small but significant increases in heart rate and blood pressure, consistent with findings from Vandrey and colleagues (2011), but contrasting with this earlier study, the magnitude of these changes was not clinically significant for any individual, most likely because high blood pressure was an exclusion criterion. Thus, among normotensive marijuana smokers, both marijuana withdrawal and the effects of nabilone appear safe with regard to cardiovascular measures.
Consistent with its FDA-approval for appetite enhancement, nabilone had marked effects on food intake. Marijuana withdrawal is characterized by an often dramatic decrease in food intake (eg, Haney et al, 2010; Cooper et al, 2012): caloric intake in the current sample dropped by almost 1500 kcal/day compared with ongoing marijuana use. Also consistent with these earlier studies, marijuana withdrawal shifted the types of foods consumed, with a higher proportion of calories derived from carbohydrates and less from fat relative to non-abstinent conditions. Nabilone (6 mg/day) returned total caloric intake to baseline levels, yet the BID condition (8 mg/day) increased caloric intake by over 20% of active marijuana use. Both nabilone doses also normalized the proportion of calories derived from each macronutrient, yet increased the amount of calories consumed at each eating occasion compared with non-abstinence. Correspondingly, maintenance on either nabilone dose increased body weight compared with placebo. For dronabinol, tolerance developed to these appetite-enhancing effects (Bedi et al, 2010), but whether this also occurs with nabilone requires further study.
In terms of potential abuse liability, acute nabilone administration (3–8 mg) has been shown to reliably increase positive mood ratings, for example, ‘good drug effect’ in nonabstinent marijuana smokers (Lile et al, 2010, 2011; Bedi et al, 2012), contrasting with uncomfortable intoxication (anxiety, altered perception) reported from nabilone administration (1–3 mg) to non-marijuana smokers (see Ben Amar, 2006; Kalliomäki et al, 2012). Yet, participants in this study reported few subjective effects from nabilone. Relative to placebo, they did not report liking or wanting to take nabilone and did not indicate that it made them feel ‘high’ or ‘tired.’ This was surprising but may be due to the fact that participants were undergoing marijuana abstinence, unlike the acute administration studies. Tolerance to nabilone’s subjective effects can develop (Lemberger et al, 1982), yet subjective ratings did not differ from placebo even on the first day of nabilone administration. Note, ratings of ‘high’ from placebo capsules were elevated in this study (nearly 20% of maximum score), we suspect because of expectancy effects: capsule administration was double-blind yet participants experienced each dosing condition so they may have come to expect that the capsule would produce effects. Typically, placebo capsules produce lower ratings of intoxication so the effects of nabilone (Bedi et al, 2012) or dronabinol (Haney et al, 2008) administration on ratings of intoxication are apparent.
Yet even if marijuana smokers experience some positive subjective effects from nabilone, there is little to suggest that a long-acting, slow-onset oral medication will have abuse liability approaching that of smoked marijuana. Although nabilone had mixed effects in one purported measure of reinforcement (Multiple-Choice Procedure; Lile et al, 2010, 2011), no participants chose nabilone when offered a choice between marijuana (1.83% THC), nabilone (2 mg), dronabinol (17.5 mg), or placebo: 78% chose marijuana (Mendelson and Mello, 1984). Further, as with dronabinol, there is little evidence of nabilone diversion or any street value despite being available for 30 years (Ware and St Arnaud-Tempe, 2010).
To conclude, nabilone robustly and selectively decreased marijuana withdrawal and relapse in the laboratory, with no concurrent increase in measures of sedation or intoxication, strongly supporting its further testing as a potential treatment medication for marijuana dependence. Given that nabilone administration was initiated during marijuana abstinence, several days before the opportunity to resume active marijuana use, this study suggests that nabilone shows promise as a relapse prevention strategy (John Mariani, Frances Levin, personal communication, 9/12). Further studies are needed to determine whether nabilone: (1) similarly prevents marijuana relapse in a clinical setting, and (2) would also be effective for abstinence initiation, in which medication is administered while marijuana use in ongoing.
References
Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J et al (2012). Quantifying the clinical significance of cannabis withdrawal. PLoS One 7: e44864.
Bedi G, Cooper Z, Haney M (2012). Subjective, cognitive, and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addiction Biol e-pub ahead of print 19 January 2012. doi: 10.1111/j.1369-1600.2011.00427.x.
Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD et al (2010). Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study. Psychopharmacology (Berl) 212: 675–686.
Ben Amar M (2006). Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol 105: 1–25.
Budney AJ, Moore BA, Rocha HL, Higgins ST (2006). Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol 74: 307–316.
Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B (2007). Oral delta-9 tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend 86: 22–29.
Carroll KM, Nich C, Lapaglia DM, Peters EN, Easton CJ, Petry NM (2012). Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less. Addiction 107: 1650–1659.
Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL (2008). The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend 96: 1–15.
Compton W, Thomas Y, Stinson FS, Grant B (2007). Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States—results from the National Epidemiologic Survey of Alcohol and Related conditions. Arch Gen Psychiatry 64: 566–576.
Cooper ZD, Foltin RW, Hart CL, Vosburg SK, Comer SD, Haney M (2012). A controlled human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addiction Biol e-pub ahead of print 28 June 2012. doi: 10.1111/j.1369-1600.2012.00461.x.
Copeland J, Swift W, Roffman R, Stephens R (2001). A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J Subst Abuse Treat 21: 55–64.
EMCDDA (2009). Annual Report on the State of the Drugs Problem in Europe www.emcdda.europa.eu/attachements.cfm/att_93236_EN_EMCDDA_AR2009_EN.pdf.
Evans SM, Foltin RW, Levin FR, Fischman MW (1995). Behavioral and subjective effects of DN-2327 (pazinaclone) and alprazolam in normal volunteers. Behav Pharmacol 6: 176–186.
Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD (1987). Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav 28: 459–464.
Foltin RW, Haney M, Comer SD, Fischman MW (1996). Effects of fenfluramine in food intake, mood, and performance of humans living in a residential laboratory. Physiol Behav 59: 295–305.
Fraser AD, Meatherall R (1989). Lack of interference by nabilone in the EMIT d.a.u. cannabinoid assay, Abbott TDx cannabinoid assay, and a sensitive TLC assay for delta 9-THC-carboxylic acid. J Anal Toxicol 13: 240.
Glass RM, Uhlenhuth EH, Hartel FW (1979). The effects of nabilone, a synthetic cannabinoid, on anxious human volunteers [proceedings]. Psychopharmacol Bull 15: 88–90.
Glass RM, Uhlenhuth EH, Hartel FW, Schuster CR, Fischman MW (1981). Single-dose study of nabilone in anxious volunteers. J Clin Pharmacol 21: 383S–396S.
Hall W (2006). The mental health risks of adolescent cannabis use. PLoS Medicine 3: 159–162.
Haney M (2009). Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict Biol 14: 9–21.
Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD et al (2012). Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biological Psychiatry 73: 242–248.
Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Cooper ZD et al (2010). Effects of baclofen and mirtazapine on laboratory model of marijuana withdrawal and relapse. Psychopharmacol 211: 233–244.
Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW (2008). Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacol 197: 157–168.
Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C et al (2004). Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacol 29: 158–170.
Haney M, Spealman R (2008). Controversies in translational research: drug self-administration. Psychopharmacol 199: 403–419.
Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW (1999). Abstinence symptoms following smoked marijuana in humans. Psychopharmacol 141: 395–404.
Hart C, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW (2002). Comparison of smoked marijuana and oral delta(9)-tetrahydrocannabinol in humans. Psychopharmacol 164: 407–415.
Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE (2010) Monitoring the future national survey results on drug use, 1975-2008 Vol I. Secondary School Students. National Institute on Drug Abuse: Bethesda, MD, USA).
Kadden RM, Litt MD, Kabela-Cormier E, Petry NM (2007). Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav 32: 1220–1236.
Kalliomäki J, Philipp A, Baxendale J, Annas P, Karlsten R, Segerdahl M (2012). Lack of effect of central nervous system-active doses of nabilone on capsaicin-induced pain and hyperalgesia. Clin Exp Pharmacol Physiol 39: 336–342.
Lemberger L, Rubin A, Wolen R, DeSante K, Rowe H, Forney R et al (1982). Pharmacokinetics, metabolism and drug-abuse potential of nabilone. Cancer Treat Rev 9 (Suppl B): 17–23.
Levin FR, Mariani JJ, Brooks DJ, Xie S, Murray KA (2010). Δ9-tetrahydrocannabivarin testing may not have the sensitivity to detect marijuana use among individuals ingesting dronabinol. Drug Alcohol Depend 1: 65–68.
Lile JA, Kelly TH, Hays LR (2010). Substitution profile of the cannabinoid agonist nabilone in human subjects discriminating δ9-tetrahydrocannabinol. Clin Neuropharmacol 33: 235–242.
Lile JA, Kelly TH, Hays LR (2011). Separate and combined effects of the cannabinoid agonists nabilone and Δ9-THC in humans discriminating Δ9-THC. Drug Alcohol Depend. http://www.ncbi.nlm.nih.gov/pubmed?term=separate and combined effects of the cannabinoid aconists nabilone 116: 86–92.
Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF et al (2011). Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend 115: 120–130.
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346: 561–564.
McGilveray I (2005). Pharmacokinetics of cannabinoids. Pain Res Manag 10: 15A–22A.
Mendelson JH, Mello NK (1984). Reinforcing properties of oral delta 9-tetrahydrocannabinol, smoked marijuana, and nabilone: influence of previous marijuana use. Psychopharmacology (Berl) 83: 351–356.
MTPRG (2004). Brief treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol 72: 455–466.
Rubin A, Lemberger L, Warrick P, Crabtree RE, Sullivan H, Rowe H et al (1977). Physiologic disposition of nabilone, a cannabinol derivative, in man. Clin Pharmacol Ther 22: 85–91.
Schierenbeck T, Riemann D, Berger M, Hornyak M (2008). Effect of illicit recreational drugs upon sleep: cocaine, ecstasy, and marijuana. Sleep Med Rev 12: 381–389.
Stephens RS, Roffman RA, Curtin L (2000). Extended versus brief treatment for marijuana use. J Consult Clin Psychol 68: 898–908.
Substance Abuse and Mental Health Services Administration (SAMHSA) (2012). Results from the 2011 National Survey on Drug Use and Health: Detailed Tables, www.samhsa.gov/data/NSDUH/2011SummNatFindDetTables/NSDUH-DetTabsPDFWHTML2011/2k11DetailedTabs/Web/PDFW/NSDUH-DetTabsCover2011.pdf.
Vandrey R, Umbricht A, Strain EC (2011). Increased blood pressure after abrupt cessation of daily cannabis use. J Addict Med 5: 16–20.
Wagner FA, Anthony JC (2002). Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. Am J Epidemiol 155: 918–925.
Ware MA, Arnaud-Trempe E (2010). The abuse potential of the synthetic cannabinoid nabilone. Addiction 105: 494–503.
Acknowledgements
The US National Institute on Drug Abuse (NIDA) supported this research (DA09236) and supplied the marijuana cigarettes. We are grateful to Laura Rolfe, Christina Hadzitheodorou, and Ashley Danies for their expert assistance in data collection and to Dr Adam Bisaga for medical supervision of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Haney’s research is funded by NIDA. She and Dr Foltin have received partial salary support for an investigator-initiated study from Astra-Zeneca. Dr Haney has also served as a consultant for GW Pharmaceuticals. Dr Comer has received partial salary support from investigator-initiated studies supported by Reckitt-Benckiser Pharmaceuticals, Schering-Plough Corporation, Johnson & Johnson Pharmaceutical Research & Development, Endo Pharmaceuticals, and MediciNova. In addition, she and Dr Vosburg have received compensation from Grunenthal GmbH to conduct a meta-analysis of drug-induced subjective responses. Dr Comer has also served as a consultant to the following companies: Analgesic Solutions, BioDelivery Sciences International, Cephalon, Inflexxion, Innovative Science Solutions, Janssen, King, Neuromed, Pfizer, and Salix. Drs Cooper and Bedi declare no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Haney, M., Cooper, Z., Bedi, G. et al. Nabilone Decreases Marijuana Withdrawal and a Laboratory Measure of Marijuana Relapse. Neuropsychopharmacol 38, 1557–1565 (2013). https://doi.org/10.1038/npp.2013.54
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.54
Keywords
This article is cited by
-
Signaling-specific inhibition of the CB1 receptor for cannabis use disorder: phase 1 and phase 2a randomized trials
Nature Medicine (2023)
-
Helpful or Harmful? The Therapeutic Potential of Medications with Varying Degrees of Abuse Liability in the Treatment of Substance Use Disorders
Current Addiction Reports (2022)
-
The emergency department care of the cannabis and synthetic cannabinoid patient: a narrative review
International Journal of Emergency Medicine (2021)
-
Cannabis use and cannabis use disorder
Nature Reviews Disease Primers (2021)
-
Pharmacological management of psychoactive substance withdrawal syndrome
Drugs & Therapy Perspectives (2021)