Abstract
Body dysmorphic disorder (BDD) is characterized by preoccupation with misperceived defects of appearance, causing significant distress and disability. Previous studies suggest abnormalities in information processing characterized by greater local relative to global processing. The purpose of this study was to probe whole-brain and regional white matter network organization in BDD, and to relate this to specific metrics of symptomatology. We acquired diffusion-weighted 34-direction MR images from 14 unmedicated participants with DSM-IV BDD and 16 healthy controls, from which we conducted whole-brain deterministic diffusion tensor imaging tractography. We then constructed white matter structural connectivity matrices to derive whole-brain and regional graph theory metrics, which we compared between groups. Within the BDD group, we additionally correlated these metrics with scores on psychometric measures of BDD symptom severity as well as poor insight/delusionality. The BDD group showed higher whole-brain mean clustering coefficient than controls. Global efficiency negatively correlated with BDD symptom severity. The BDD group demonstrated greater edge betweenness centrality for connections between the anterior temporal lobe and the occipital cortex, and between bilateral occipital poles. This represents the first brain network analysis in BDD. Results suggest disturbances in whole brain structural topological organization in BDD, in addition to correlations between clinical symptoms and network organization. There is also evidence of abnormal connectivity between regions involved in lower-order visual processing and higher-order visual and emotional processing, as well as interhemispheric visual information transfer. These findings may relate to disturbances in information processing found in previous studies.
Similar content being viewed by others
INTRODUCTION
Body dysmorphic disorder (BDD) is characterized by preoccupation with misperceived defects of appearance or excessive concern about slight physical anomalies, causing clinically significant distress and impairment of functioning (American Psychiatric Association, 2000). BDD affects approximately 2% of the general population, making it more prevalent than schizophrenia or bipolar I disorder (Buhlmann et al, 2010; Koran et al, 2008). It is associated with high lifetime rates of psychiatric hospitalization (48%) (Phillips and Diaz, 1997a) and suicide attempts (22.2–27.5%) (Buhlmann et al, 2010; Phillips et al, 2005). Insight in BDD is on a continuum, with 35.6–60% of BDD patients being delusional in their convictions of disfigurement (Mancuso et al, 2010; Phillips et al, 2006). Despite its high prevalence and severity, relatively little is known about the neurobiology.
Individuals with BDD perceive details of appearance features as defective without seemingly being able to contextualize that they are minor relative to their whole appearance. Moreover, neuropsychological (Deckersbach et al, 2000) and psychophysical (Feusner et al, 2010a; Stangier et al, 2008) studies suggest greater local relative to global visual and visuospatial processing. Functional magnetic resonance imaging (fMRI) studies using own and others’ faces and inanimate object stimuli also suggest imbalances in detailed vs holistic/configural processing marked by abnormalities in primary and/or secondary visual cortical, temporal, and prefrontal systems (Feusner et al, 2007, 2010c, 2011).
Thus, there is evidence suggesting abnormalities in information processing in BDD. However, multiple interacting systems are likely responsible for neuropsychological and psychophysical performance (ie, visual perceptual systems, attentional systems, and prefrontal executive function systems); from these studies it is therefore difficult to discern which system(s) may be operating abnormally. In addition, the fMRI studies have reported regional abnormalities, rather than what is occuring on a network level, or how regions interact within larger systems to process information.
To better understand brain network organization in BDD, this study investigated structural networks using a graph-theoretical approach. This provides quantitative analyses of complex brain networks by modeling them as organizational systems, which can additionally be related to information such as clinical symptom severity. Structural connectivity patterns may predict functional connectivity patterns (Honey et al, 2009; Kotter and Sommer, 2000); thus, structural network topology may provide indirect information about functional organization in BDD. No study to date has investigated brain network organization in BDD.
Here, we seek to characterize whole brain and regional white matter network organization in individuals with BDD relative to that in healthy controls, and to relate this organization to clinical symptom severity. The phenomenology, as well as neuropsychological, psychophysical, and functional neuroimaging studies informed our hypotheses.
We hypothesized that the whole brain network organization in individuals with BDD would reflect highly localized information processing. This would manifest in: (1) highly localized subnetworks, with resultant abnormally high modularity (Fan et al, 2011); (2) less efficient transfer of information across the whole brain, resulting in lower global efficiency (Bullmore and Sporns, 2009); and (3) abnormally high mean clustering coefficient (MCC) (similar to what was previously found in a study in a related disorder, obsessive-compulsive disorder (OCD) (Zhang et al, 2011)). Additionally, we hypothesized that BDD symptom severity and poor insight/delusionality would positively correlate with mean CC and modularity, and negatively correlate with global efficiency.
We also predicted abnormalities in the BDD group in regions involved in visual and emotional processing, and in frontostriatal systems. First, as informed by a previous fMRI study showing hypoactivity in dorsal visual stream regions (Feusner et al, 2007) (which contribute to holistic/configural visual processing), we predicted lower connectivity of nodes in the dorsal visual stream with other nodes in the brain. Specifically, the BDD group would have lower node degree in the superior parietal lobule, lateral occipital cortex, cuneus, supramarginal gyrus, and angular gyrus (Creem and Proffitt, 2001). Second, we hypothesized lower node degree in the left lingual gyrus, left occipital pole, and left occipital fusiform gyrus, which are regions found to be hypoactive in a previous study of own-face processing (Feusner et al, 2010c). Third, owing to the finding in that study of hyperactivity within frontostriatal circuits (orbitofrontal cortex (OFC) and the caudate), we predicted higher node betweenness centrality. This would reflect greater influence of these frontostriatal regions with respect to the whole network; we postulated that this would be due to a dominant effect on the network of engagement of obsessive thoughts and compulsive behaviors, which were previously found to correlate with activity in these regions (Feusner et al, 2010c).
Finally, we hypothesized lower edge betweenness centrality for node pairs connecting regions in the anterior temporal lobe with regions in the occipital lobe, and node pairs connecting right and left occipital cortices. These hypotheses were informed by findings from a previous diffusion tensor imaging (DTI) study in which fiber disorganization in the inferior longitudinal fasciculus (ILF) and forceps major (FM) correlated with the clinical symptom of poor insight (Li et al, 2010). (For a more detailed description of these graph theory metrics, see Supplementary Materials and methods.)
MATERIALS AND METHODS
Participants
The UCLA Office of Human Research Protection Program approved the study protocol. In all, 14 unmedicated participants with BDD and 16 healthy controls, ages 20–48 years old, provided informed consent and were enrolled. BDD and control participants of equivalent gender, age, and level of education were recruited from the community. All had previously participated in a prior fMRI study of own-face processing (Feusner et al, 2010b). All were right-handed, as determined through the Edinburgh Handedness Inventory (Oldfield, 1971). Diagnoses were made by JDF, who has clinical expertise with this population, using the BDD Module (Phillips, 1995), a reliable diagnostic module modeled after the Structured Clinical Interview for DSM-IV. In addition, we performed a comprehensive clinical psychiatric evaluation and screened BDD and healthy control participants for comorbid Axis I disorders with the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al, 1998).
The following served as exclusion criteria: substance abuse and/or dependence within the past 12 months, lifetime neurological disorder, pregnancy, or any current medical disorder that may affect cerebral metabolism. We excluded BDD participants with any concurrent axis I disorder besides dysthymic disorder, major depressive disorder (MDD), or generalized anxiety disorder (GAD). Depression and anxiety are frequently comorbid in BDD, and thus a sample excluding these would not be representative. However, we required that BDD be the primary diagnosis as defined by the MINI. Healthy controls could not have any current or past axis I disorder. To assess BDD symptom severity, we administered the BDD version of the Yale–Brown Obsessive-Compulsive Scale (BDD-YBOCS) (Phillips et al, 1997b), a validated scale widely used to evaluate symptom severity in BDD, with scores ranging from 0 to 48. To assess insight and delusionality, we administered the Brown Assessment of Beliefs Scale (BABS), a validated scale with scores ranging from 0 to 24 (Eisen et al, 1998). Higher BABS scores index poorer insight. Last, we used the 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960), and the Hamilton Anxiety Rating Scale (HARS) (Hamilton, 1969), both widely used and well-validated scales, to measure depressive and anxiety symptoms, respectively.
All BDD participants were required to have a score of ⩾20 on the BDD-YBOCS, were free from psychotropic medications for a minimum of 8 weeks before study entry, and were not receiving cognitive-behavioral therapy.
Imaging Data Acquisition
We scanned participants using a 3 T Allegra MRI scanner (Siemens Medical Solutions USA Inc., Malvern, Pennsylvania). Diffusion-weighted MR imaging data using single-shot spin-echo echo-planar imaging were acquired using the following parameters: field of view=240 mm; voxel size=2.5 × 2.5 × 3.0 mm3, with 0.75 mm gap; TR/TE=7400/96 ms; and flip angle 9°. We collected 44 contiguous axial slices aligned to the anterior commissure–posterior commissure line along 34 gradient-sensitizing directions with b=1000 s/mm2 and one minimally diffusion-weighted scan. In addition, high-resolution structural images were acquired using T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) with the following parameters: sagittal slicing; TR=2300 ms; TE=293 ms; matrix=256 × 256; 160 slices; 0.5 mm gap; field of view=256 × 256 × 160 mm3; flip angle=8°; and voxel size=1.3 × 1.3 × 1.0 mm3.
Data Processing
Figure 1 illustrates the data processing steps.
Structural network analysis processing. (a) High-resolution T1 magnetization-prepared rapid gradient echo (MP-RAGE) of an individual example participant. (b) In all, 113 cortical and subcortical regions of interest (ROIs), covering the whole brain, from the Harvard–Oxford probabilistic atlas. (c) Diffusion-weighted magnetic resonance imaging (MRI). (d) Whole brain tractography. (e) ROIs were registered to each participant’s diffusion tensor imaging (DTI) space, and served as nodes from which the number of streamlines between them was identified. (f) A total of 113 × 113 weighted matrix and (g) 113 × 113 binarized matrix. (h) Network render of an individual participant imposed on the Montreal Neurological Institute (MNI) standard brain (for visualization purposes this only shows the top 6% strongest connections).
Calculation of diffusion tensors
All DTI data were corrected for eddy current and motion distortions using FSL (http://www.fmrib.ox.ac.uk/fsl/fdt/fdt_eddy.html), and the gradient table was updated based on the computed rotation matrix. We used DTIFIT in FSL (http://www.fmrib.ox.ac.uk/fsl/fdt/fdt_dtifit.html) to fit a diffusion tensor at each voxel.
DTI deterministic tractography
We computed whole-brain deterministic DTI tractography using Diffusion Toolkit (http://trackvis.org/blog/tag/diffusion-toolkit/). We reconstructed white matter fiber tracts by seeding at every voxel in the brain and applying the Fiber Assignment by Continuous Tracking (FACT) algorithm (Mori and van Zijl, 2002) with a maximum turn angle of 35°. Cortical and subcortical regions of interest (ROIs) were defined using the Harvard–Oxford cortical and subcortical probabilistic atlases (Desikan et al, 2006). All midline cortical masks were bisected to define separate hemispheric ROIs for each cortical region. The masks were set to a liberal probabilistic threshold of 10% to allow for the inclusion of tissue along the gray–white matter interface, where DTI tractography estimates are most reliable (Morgan et al, 2009). We used FSL’s FLIRT program (Jenkinson et al, 2002) to determine the optimal transformation between each participant’s DTI volume and the corresponding MP-RAGE (12 degree-of-freedom (d.f.) affine registration with a mutual information-based cost function), as well as between each participant’s MP-RAGE and the MNI152 T1 average brain (on which the Harvard-Oxford probabilistic atlases are based). We then combined the obtained two transformations to yield a final transformation, which was subsequently inverted and applied to register the 113 ROIs (in the atlas space) to each participant’s DTI space. To assure that ROI masks did not overlap after registration, each voxel was uniquely assigned to the mask for which it had the highest probability of membership.
Matrix construction
For each pair of ROIs, we determined the number of fibers connecting them. A fiber was considered to connect two ROIs if it originated in the first ROI and terminated in the second, or vice versa. We repeated this process for all possible pairs to determine the whole brain fiber connectivity matrix. These matrices served as the input for subsequent brain network analyses (Rubinov and Sporns, 2010). We assessed the matrices at 11 different sparsity levels (defined as the existing number of edges in a graph divided by the maximum possible number of edges) from 10 to 20%, at intervals of 1%. (For further description of, and rationale for, this sparsity thresholding, see Supplementary Materials and methods.) We then binarized the thresholded matrices to create corresponding brain network adjacency matrices, where 1 represents a connection and 0 represents no connection.
Graph Theory Metrics
The following is a brief description of the graph theory metrics used in this study. (For further descriptions, see Supplementary Materials and methods and Bullmore and Sporns (2009)) In graph theory, a network is comprised of ‘nodes’ (here, anatomically defined ROIs) and the connections or ‘edges’ between them (in this case, the white matter tracts). Node degree is the number of total nodes in the network that have direct connections to that node; a high value thus signifies that this node is highly connected to other nodes in the network. The CC of a node is the ratio of the number of actual connections among its first-degree neighbors to the number of all possible connections. Thus, a high CC value for a node indicates that its neighbors are strongly interconnected to one another. MCC is the average of the CC for all nodes in the network; high values may confer greater local efficiency of information transfer of a network (Bullmore and Sporns, 2009). Globally efficiency is mathematically defined by averaging the inverse shortest path lengths across all node pairs. (Path length is computed by counting the minimum number of intermediate nodes needed to pass through to link any node pair.) A high global efficiency value represents a high overall capacity for parallel information transfer and integrated processing (Bullmore and Sporns, 2012). Modularity measures how strongly nodes in a community interconnect in comparison to a random graph. Thus, the higher the modularity value for a given community structure, the less likely it is to be the result of chance alone. Node betweenness centrality is the fraction of all shortest paths that contain a specific node. Thus, higher values indicate that a node has more ‘influence’ over flow of information between other nodes, in networks in which information tends to follow the shortest available path (Girvan and Newman, 2002). Similarly, edge betweenness centrality is the fraction of all shortest paths in the network that contain this connection. Higher values indicate a connection that has greater influence over other connections in the network.
We analyzed these connectivity matrices using the Brain Connectivity Toolbox (https://sites.google.com/a/brain-connectivity-toolbox.net/bct/) to yield the graph theory metrics of interest. For each metric we evaluated the area under the curve (AUC) over a range of sparsities to provide summarized measures of the network. We calculated both local metrics (for specific nodes) and global metrics (averaged across all nodes).
Three global network metrics were of primary interest based on the hypotheses of this study: CC, global efficiency, and modularity. Additionally, three local network metrics were of primary interest: node degree, node betweenness centrality, and edge betweenness centrality. For node degree we examined nodes in the dorsal visual stream (superior parietal, lateral occipital, cuneus, supramarginal gyrus, and angular gyrus) (Creem and Proffitt, 2001), as well as the left lingual gyrus, left occipital pole, and left occipital fusiform gyrus. For node betweenness centrality, we examined the OFC and caudate.
For edge betweenness centrality, we examined sets of connections between visual and emotional processing systems. These included node pairs between the anterior temporal lobe (temporal pole, amygdala, and hippocampus) and the occipital lobe (occipital fusiform, temporal occipital fusiform, and occipital pole), which approximates white matter connections via the ILF (Catani and Schotten, 2008). We also examined the node pair of the right and left occipital cortex (right and left occipital pole), which approximates connections via the FM (Catani and Schotten, 2008).
Statistical Analyses
We conducted statistical analyses on age- and gender-corrected data using General Linear Model Univariate in SPSS, with gender as a fixed factor and age as continuous predictor.
For global network metrics, we performed two-tailed two-sample t-tests to compare MCC, global efficiency, and modularity AUC values between the healthy control and BDD groups. Because these metrics are non-independent, with respective separate hypotheses for each, we analyzed these separately rather than implementing an omnibus test or correcting for multiple comparisons. We used Pearson’s correlation coefficients to assess the association between the global network metrics and BDD-YBOCS and BABS scores in the BDD group. We used a significance threshold of α=0.05, Bonferroni corrected for multiple comparisons.
For the local network metrics, we performed repeated-measures ANOVA to compare separately each graph theory metric of node degree, node betweenness centrality, and edge betweenness centrality values between the healthy control and BDD groups; group was one factor, and each node (or node pair for edge betweenness centrality) was the repeated-measures factor. We used Huynh–Feldt adjustments for non-sphericity.
RESULTS
Demographics and Psychometrics
All BDD participants had preoccupations with perceived facial defects. Two had comorbid GAD, one had comorbid MDD, and three had both GAD and MDD or dysthymia (Table 1).
Global Network Results
The BDD group showed significantly higher MCC than controls across the range of sparsities (aside from at 17%) (Figure 2a), and for the AUC (5.14±0.071 vs 5.05±0.074, t=3.5, d.f.=28, Cohen’s d=1.32, P=0.0015) (Figure 2d). There were no significant differences between groups for any of the sparsity values or AUC for modularity (AUC: 4.3±0.11 for BDD, 4.3±0.12 for controls, t=−0.12, d.f.=28, P=0.90) (Figures 2b and e) or global efficiency (AUC: 5.08±0.02 for BDD, 5.09±0.03 for controls, t=−0.71, d.f.=28, P=0.48) (Figures 2c and f).
Global network measures in body dysmorphic disorder (BDD) and healthy control groups. Graphs in the top row show between-group differences as a function of network sparsity threshold for: (a) mean clustering coefficient (MCC); (b) modularity; and (c) global efficiency. Graphs in the bottom row show between-group differences for the area under the curve (AUC) for the range of sparsities tested for: (d) MCC; (e) modularity; and (f) global efficiency.
Correlation with Clinical Variables
There was a significant negative correlation between global efficiency and BDD-YBOCS scores (r=−0.68, P=0.0069) (Figure 3). Modularity positively correlated with BABS scores (r=0.54, P=0.047), although it did not survive Bonferroni correction. There were no significant correlations between MCC and BDD-YBOCS (r=0.26, P=0.37) or BABS (r=−0.074, P=0.80) scores, between global efficiency and BABS (r=−0.24, P=0.40), or between modularity and BDD-YBOCS scores (r=0.15, P=0.62).
Correlations between clinical severity measures and global network measures in individuals with body dysmorphic disorder (BDD). The top row shows correlations between the BDD version of the Yale–Brown Obsessive Compulsive Scale (BDD-YBOCS) scores and area under the curve (AUC) values for: (a) mean clustering coefficient (MCC); (b) modularity; and (c) global efficiency. The bottom row shows correlations between the Brown Assessment of Beliefs Scale (BABS) scores (a measure of poor insight/delusionality) and AUC values for: (d) MCC; (e) modularity; and (f) global efficiency.
As a post hoc analysis, we explored separate correlations between the network metrics and the items of the BDD-YBOCS that index obsessional thoughts (items 1–5) and the items that index behaviors (compulsive-like and avoidant—items 6–10 and 12). There was a significant negative correlation between behaviors and global efficiency (r=−0.70, P=0.0047), but the correlation between obsessive thoughts and global efficiency was not significant (r=−0.48, P=0.081). There were no significant correlations with MCC and modularity for these subscale measures.
We also conducted post hoc correlation analyses to test the relationship between the network metrics and depression severity. (We did not test relationships with anxiety severity separately, as the correlation between HARS and HDRS scores in our sample was r=0.93) There were no significant correlations between HDRS scores and modularity (r=−0.46, P=0.098), global efficiency (r=0.036, P=0.90), or MCC (r=0.34, P=0.23).
Local (Nodal) Network Results
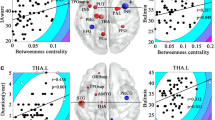
The analysis of edge betweenness centrality revealed a significant group effect (F1,28=4.22, P=0.049) and node effect (F10.57,296=20.39, P<0.0001), but no group by node effect (F10.57,296=1.15, P=0.33). Post hoc two-sample t-tests revealed that the BDD group showed significantly higher edge betweenness centrality for the connection between left temporal pole and left occipital pole (BDD–CON=60.44, t=3.73, d.f.=28, Cohen’s d=1.4, P=0.00086), the connections between left temporal pole and left temporal occipital fusiform cortex (BDD–CON=23.76, t=2.14, d.f.=28, Cohen’s d=0.8, P=0.041), between left amygdala and left occipital pole (BDD–CON=30.15, t=2.2, d.f.=28, Cohen’s d=0.83, P=0.036), and between right amygdala and right occipital pole (BDD–CON=15.15, t=2.13, d.f.=28, Cohen’s d=0.8, P=0.042). Only the connection between left temporal pole and left occipital pole survived Bonferroni correction, using a corrected α threshold of 0.05/18=0.0028 (accounting for all 18 possible node connections for the right and left between the anterior temporal lobe and the visual cortex). The BDD group showed significantly higher edge betweenness centrality for the connection between left and right occipital pole (BDD–CON=112.39, t=2.12, d.f.=28, Cohen’s d=0.8, P=0.043).
There were no significant differences between groups for either node degree or node betweenness centrality (Supplementary Table S1).
We additionally conducted post hoc correlation analyses between edge betweenness centrality values and BDD-YBOCS scores and BABS scores for the connections that were significantly different between groups (before correction for multiple comparisons). For the connection between the left temporal pole and the left temporal occipital fusiform cortex, there was a significant correlation between edge betweenness centrality and total BDD-YBOCS scores (r=0.73, P=0.0031), which survived Bonferroni correction (α threshold of 0.05/10=0.005). For this connection, there were significant correlations between both the BDD-YBOCS obsessive thoughts items and the behaviors items, and edge betweenness centrality (r=0.71, P=0.0043 and r=0.72, P=0.0039, respectively). There was also a correlation between behaviors and edge betweenness centrality for the left temporal pole/left occipital fusiform cortex connection (r=0.56, P=0.036) (not surviving Bonferroni correction). The correlation between obsessive thoughts and edge betweenness centrality for this connection was not significant (r=0.46, P=0.098).
Additional Analyses
We additionally performed post hoc analyses to explore if prior treatment in BDD subjects affected results. Eight of the BDD subjects were treatment naïve, and six had received prior medication treatment. (Two subjects received brief psychotherapy, but it was unrelated to BDD.) The previously medicated group showed significantly higher MCC than controls (AUC: 5.19±0.061 vs 5.05±0.074, t=4.09, d.f.=20, Cohen’s d=1.83, P=0.00057), while the BDD medication naïve group showed a trend for higher MCC than controls (AUC: 5.11±0.059 vs 5.05±0.074, t=3.5, d.f.=22, Cohen’s d=0.83, P=0.064).
For the local (nodal) results, there were no significant differences between groups when we separately analyzed the BDD medication naïve and the previously medicated BDD groups, each compared with matched sets of healthy controls. (This may have been due to loss of power in these smaller subgroups.)
DISCUSSION
This study represents the first brain network analysis in BDD. Individuals with BDD exhibit abnormal white matter brain network organization, as characterized by higher MCC compared with controls. In addition, global efficiency negatively correlates with BDD symptom severity. Individuals with BDD also demonstrate higher edge betweenness centrality for connections between anterior temporal and occipital regions, as well as between bilateral occipital poles.
Global Metrics
As hypothesized, individuals with BDD have higher MCC relative to controls, suggesting a disturbance in network organization. In general, higher CCs are found in networks with a more regular, as opposed to random, organization (Stam and Reijneveld, 2007). Higher CCs are thought to confer locally higher degree of information transfer (Bullmore and Sporns, 2009). However, such networks that are overall more regular may also exhibit globally reduced signal propagation speed, computational power, and synchronizability across distant regions (Watts and Strogatz, 1998). As structural and functional network organizations in the brain share many topological features (Honey et al, 2010) (and structural connectivity patterns may predict functional connectivity patterns; Honey et al (2009), Honey et al (2010), and Kotter and Sommer (2000)), such disturbance in structural network organization may provide indirect information about functional organization in BDD.
Higher MCC in BDD suggests a network organization in which local connections dominate. One possible clinical implication of this could be an imbalance in global and local processing of visual information, leading to a distorted perception of appearance; individuals with BDD perceive detailed imperfections and flaws (local information) and are unable to contextualize them as minor relative to their whole appearance. This is consistent with previous findings in BDD that provide evidence of greater local (relative to global) visual and visuospatial information processing (Deckersbach et al, 2000; Feusner et al, 2007, 2010a, 2010c, 2011). Specifically, a previous neuropsychological study that included a measure of visuospatial construction and memory (Rey Osterrieth Complex Figure Test) demonstrated poor performance in the BDD group, mediated by poor organizational strategies due to selective recall of details instead of larger design features (Deckersbach et al, 2000). A study examining the face inversion effect found that individuals with BDD, relative to healthy controls, demonstrated less delay in response time when identifying upside-down relative to upright faces, suggesting a tendency to engage in highly detailed processing of faces regardless of their orientation. In contrast, healthy controls are more likely to engage in holistic processing of upright faces, yet rely on (slower) detailed processing of inverted faces. Another psychophysical experiment in BDD found an advantage for change detection for facial features of others’ faces (Stangier et al, 2008).
However, it is unclear if abnormal performance in these studies was the result of impairments in information processing at the level of executive functioning resulting in poor memory organization strategies, selective attention, visual integration, or lower-order detail and/or holistic/configural visual processing. Higher MCC found in this study could reflect abnormal network organization across the whole brain, and could relate to these abnormalities in information processing; however, future studies are necessary to test this directly.
Although no previous brain network studies have been performed in BDD, one study examined functional network properties in OCD (Zhang et al, 2011). Relative to healthy controls, individuals with OCD demonstrated a pattern of significantly greater CCs, but not significantly different shortest path lengths. However, this was only found in a ‘top-down control network’ (multiple prefrontal, parietal, temporal, occipital, and subcortical regions) but not for whole-brain functional networks. The study also found that CC correlated with functional connectivity for primarily short-range functional connections. BDD has similarities to OCD in terms of overlapping phenomenology, shared heredity, and evidence of shared genetics (Hollander and Wong, 1995; Monzani et al, 2012). Although this study in OCD (Zhang et al, 2011) examined functional rather than structural networks, the current study suggests that BDD individuals may show similar patterns of aberrant structural network properties.
In this study, the previously medicated group had greater differences in MCC relative to healthy controls than the medication naïve group did. A possible explanation for this is that individuals who were more severely ill, and hence had more aberrant brain network organization, were more likely to have been medicated in the past.
Local Metrics
Contrary to our hypotheses, we found greater edge betweenness centrality for connections between temporal pole and occipital pole nodes. White matter tracts connecting these regions are considered part of the ILF (Catani and Schotten, 2008). Significantly greater edge betweenness centrality was observed for nodes that span early (eg, V2) visual processing systems with higher-order visual processing systems in the temporal pole. The temporal pole is thought to be involved in integration of sensory, motor, and linguistic information with semantic knowledge and has been proposed to represent a hub in a cortical semantic network (Patterson et al, 2007). Because edge betweenness centrality quantifies the fraction of all shortest paths in a graph containing the given edge, greater values in BDD between occipital and anterior temporal regions suggests that this connection is more influential on the whole brain network in individuals with BDD compared with controls. This may also be indicative of heightened communication between these nodes.
A relevant clinical implication of this is that visual information processing for individuals with BDD may interfere or ‘bleed into’ many cognitive processes. This is consistent with the observation that for individuals with BDD a very large proportion of their time (on average 3–8 h per day (Phillips, 2005)) is occupied by intrusive, obsessive thoughts, usually relating to the visual perception of their appearance. The fact that the finding in this study was significant on the left may point to a more dominant left hemisphere involvement in visual information processing in BDD, consistent with existing evidence for greater detail and analytic processing found in a previous fMRI study (Feusner et al, 2007).
There are alternative interpretations of the findings involving the temporal pole, as it subserves multiple functions. For example, a recent study in pathological gambling found relationships between activity in the temporal poles and both gambling urges and subjective emotional responses (Balodis et al, 2012). Given previously described functions of the temporal pole, the authors offered the possible explanation that affectively salient gambling cues may have triggered the retrieval of personally relevant emotional memories. A similar process may occur in individuals with BDD, but in this case visual appearance cues may be the typical trigger of such emotional memories.
Contrary to our hypotheses, we found greater edge betweenness centrality for the connection between bilateral occipital poles. This connection likely approximates the portion of the FM that connects relatively early visual processing regions of the right and left visual cortical hemispheres (Putnam et al, 2010). Although the significance of this finding relative to the phenomenology of BDD is not entirely clear, a possible explanation is that preoccupations with perceived defects in BDD may rely heavily on visual processing, for which early communication between bilateral visual fields is generally important.
Correlations with Clinical Variables
In the BDD group, there was a significant negative correlation between global efficiency and BDD-YBOCS. Greater severity of symptoms is thus associated with lower global integration of the network. The results for the separate correlation analyses with the obsessive thoughts and the behaviors items of the BDD-YBOCS suggest that the relationship between BDD symptom severity and global efficiency is driven to a greater extent by the severity of compulsive/avoidant behaviors than by obsessive thoughts. This suggests that BDD symptomatology (in particular compulsive and avoidant behaviors) globally relates to a multitude of brain subsystems. The overall effect of such interactions may be to impact negatively the efficiency of the nervous system.
Interestingly, despite this negative correlation, we did not detect a significant between-group difference in global efficiency. We posit that phenotypes, such as propensity for obsessive thoughts and compulsive behaviors, may map better to brain pathophysiology than DSM or ICD-10 diagnostic categories. Categorical diagnostic constructs in psychiatry, such as the diagnosis of BDD, may encompass multiple overlapping endophenotypes and phenotypes (Insel and Cuthbert, 2009), particularly as they may represent heterogeneous groupings of symptom clusters or dimensions.
Secondly, on a local level the strong correlation between edge betweenness centrality and BDD-YBOCS scores, for the connection between the left temporal pole and the left temporal occipital fusiform cortex, is an indication that individuals with greater severity of symptoms tend to have a larger percentage of all shortest paths that include this connection. As this connection is likely facilitated by the ILF, this strong correlation thus adds to a growing literature supporting the role of the ILF in feed-forward processes, which may involve consolidation of visual memories (Shinoura et al, 2007). Additionally, evidence also supports its involvement in feedback information processing, carrying signals regarding emotional valence of stimuli to the visual cortex and resulting in enhanced visual processing (Morris et al, 1998).
Moreover, evidence from non-human primates and humans suggests that the temporal pole is involved in linking highly processed perceptual information with emotional responses, which contributes to the formation of personal semantic memory (Olson et al, 2007). Thus, the degree to which those with BDD experience intrusive, obsessive thoughts and compulsive behaviors may be associated with the proportion of general information transfer throughout the brain that includes this connection, which is involved in integrating emotion and memory with visual processing systems.
A clinical implication of this is that in individuals with BDD, obsessive thoughts about appearance and, especially, compulsive behaviors and urges to engage in such behaviors, may be tightly linked with what they perceive visually. This, in turn, is influenced by visually related memories and emotion. Additionally, as this finding manifests with a statistically significant laterality (to the left side), a greater degree of detailed analytic visual processing may thus be associated with greater symptom severity (Evans et al, 2000). As such, in the future we plan to explore the possibility that this connection may represent an imaging biomarker for an important phenotype in BDD.
Limitations
Small sample size may have resulted in decreased ability to detect significant differences with smaller effect sizes. A subset of the BDD group had comorbid GAD and/or a depressive disorder, which may have been a confound. Although we excluded substance abuse or dependence, we did not assess for tobacco use. Another limitation is that IQ measurements of participants were not available. Other studies have found IQ to be positively correlated with global efficiency in white matter networks (Li et al, 2009), and negatively correlated with path length (although not with MCC) in functional networks (van den Heuvel et al, 2009). In our study, the groups did not significantly differ on total years of education. Moreover, previous neuropsychological studies have not found abnormal IQ in individuals with BDD (Deckersbach et al, 2000; Dunai et al, 2010; Hanes, 1998).
In addition, there are inherent limitations in diffusion tractography (Jbabdi and Johansen-Berg, 2011). For example, such analyses may be dependent on the choice of anatomical atlas, which subsequently determine the choice the nodes (Wang et al, 2009; Zalesky et al, 2010). The 12-parameter affine transformations we used for realigning the MP-RAGE and DTI spaces only partially corrects for B0 inhomogeneity-induced geometric distortions; alternative techniques using nonlinear registration may better address such distortions for future studies.
CONCLUSIONS
Individuals with BDD show disturbances in topological organization of structural networks, which correlate with clinical symptomatology. In addition, there is evidence of abnormal connectivity in regions involved in interhemispheric visual information transfer, and those involved in lower- and higher-order visual and emotional processing, the latter of which also correlates with clinical symptomatology. These findings may be associated with disturbances in information processing found in previous studies. Future studies of individuals earlier in the course of illness (adolescence) and in unaffected first-degree relatives will be useful to determine if these findings represent endophenotypes predisposing to specific clinical symptoms in BDD.
References
American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR 4th edn. American Psychiatric Association: Washington, DC.
Balodis IM, Lacadie CM, Potenza MN (2012). A preliminary study of the neural correlates of the intensities of self-reported gambling urges and emotions in men with pathological gambling. J Gambl Stud 28: 493–513.
Buhlmann U, Glaesmer H, Mewes R, Fama JM, Wilhelm S, Brahler E et al (2010). Updates on the prevalence of body dysmorphic disorder: a population-based survey. Psychiatr Res 178: 171–175.
Bullmore E, Sporns O (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10: 186–198.
Bullmore E, Sporns O (2012). The economy of brain network organization. Nat Rev Neurosci 13: 336–349.
Catani M, Schotten Td (2008). A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44: 1105–1132.
Creem SH, Proffitt DR (2001). Defining the cortical visual systems: ‘What’, ‘Where’, and ‘How’. Acta Psychol (Amst) 107: 43–68.
Deckersbach T, Savage C, Phillips K, Wilhelm S, Buhlmann U, Rauch S et al (2000). Characteristics of memory dysfunction in body dysmorphic disorder. J Int Neuropsychol Soc 6: 673–681.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31: 968–980.
Dunai J, Labuschagne I, Castle DJ, Kyrios M, Rossell SL (2010). Executive function in body dysmorphic disorder. Psychol Med 40: 1541–1548.
Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA (1998). The Brown Assessment of Beliefs Scale: reliability and validity. Am J Psychiatry 155: 102–108.
Evans MA, Shedden JM, Hevenor SJ, Hahn MC (2000). The effect of variability of unattended information on global and local processing: evidence for lateralization at early stages of processing. Neuropsychologia 38: 225–239.
Fan Y, Shi F, Smith JK, Lin W, Gilmore JH, Shen D (2011). Brain anatomical networks in early human brain development. NeuroImage 54: 1862–1871.
Feusner JD, Hembacher E, Moller H, Moody TD (2011). Abnormalities of object visual processing in body dysmorphic disorder. Psychol Med 41: 2385–2397.
Feusner JD, Moller H, Altstein L, Sugar C, Bookheimer S, Yoon J et al (2010a). Inverted face processing in body dysmorphic disorder. J Psychiatr Res 44: 1088–1094.
Feusner JD, Moody T, Hembacher E, Townsend J, McKinley M, Moller H et al (2010b). Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Arch Gen Psychiatry 67: 197–205.
Feusner JD, Moody T, Townsend J, McKinley M, Hembacher E, Moller H et al (2010c). Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Arch Gen Psychiatry 67: 197–205.
Feusner JD, Townsend J, Bystritsky A, Bookheimer S (2007). Visual information processing of faces in body dysmorphic disorder. Arch Gen Psychiatry 64: 1417–1425.
Girvan M, Newman ME (2002). Community structure in social and biological networks. Proc Natl Acad Sci USA 99: 7821–7826.
Hamilton M (1969). Diagnosis and rating of anxiety. Br J Psychiatry 3: 76–79.
Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62.
Hanes K (1998). Neuropsychological performance in body dysmorphic disorder. J Int Neuropsychol Soc 4: 167–171.
Hollander E, Wong C (1995). Introduction: obsessive-compulsive spectrum disorders. J Clin Psychiatry 56 (Suppl 4): 3–6.
Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R et al (2009). Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci 106: 2035–2040.
Honey CJ, Thivierge J-P, Sporns O (2010). Can structure predict function in the human brain? NeuroImage 52: 766–776.
Insel TR, Cuthbert BN (2009). Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry 66: 988–989.
Jbabdi S, Johansen-Berg H (2011). Tractography: where do we go from here? Brain Connect 1: 169–183.
Jenkinson M, Bannister P, Brady M, Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17: 825–841.
Koran LM, Abujaoude E, Large MD, Serpe RT (2008). The prevalence of body dysmorphic disorder in the United States adult population. CNS Spectr 13: 316–322.
Kotter R, Sommer FT (2000). Global relationship between anatomical connectivity and activity propagation in the cerebral cortex. Philos Trans R Soc Lond Ser B 355: 127–134.
Li W, Zhang L, Arienzo D, Leow A, Feusner JD (2010). Fractional anisotropy differences of the inferior fronto-occipital fasciculus in body dysmorphic disorder. Society for Neuroscience Annual Meeting. San Diego, CA.
Li Y, Liu Y, Li J, Qin W, Li K, Yu C et al (2009). Brain anatomical network and intelligence. PLoS Comput Biol 5: e1000395.
Mancuso SG, Knoesen NP, Castle DJ (2010). Delusional versus nondelusional body dysmorphic disorder. Compr Psychiatry 51: 177–182.
Monzani B, Rijsdijk F, Iervolino AC, Anson M, Cherkas L, Mataix-Cols D (2012). Evidence for a genetic overlap between body dysmorphic concerns and obsessive-compulsive symptoms in an adult female community twin sample. Am J Med Genet B 159B: 376–382.
Morgan VL, Mishra A, Newton AT, Gore JC, Ding Z (2009). Integrating functional and diffusion magnetic resonance imaging for analysis of structure-function relationship in the human language network. PLoS One 4: e6660.
Mori S, van Zijl PC (2002). Fiber tracking: principles and strategies—a technical review. NMR Biomed 15: 468–480.
Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ et al (1998). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121 (Part 1): 47–57.
Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113.
Olson IR, Plotzker A, Ezzyat Y (2007). The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130: 1718–1731.
Patterson K, Nestor PJ, Rogers TT (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 8: 976–987.
Phillips KA (2005) The Broken Mirror Edition. Oxford University Press: New York.
Phillips KA (1995). Diagnostic Instruments for body dysmorphic disorder. American Psychiatric Association 148th Annual Meeting. Miami, FL. p 157.
Phillips KA, Coles ME, Menard W, Yen S, Fay C, Weisberg RB (2005). Suicidal ideation and suicide attempts in body dysmorphic disorder. J Clin Psychiatry 66: 717–725.
Phillips KA, Diaz SF (1997a). Gender differences in body dysmorphic disorder. J Nerv Ment Disord 185: 570–577.
Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK (1997b). A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale–Brown Obsessive Compulsive Scale. Psychopharmacol Bull 33: 17–22.
Phillips KA, Menard W, Pagano ME, Fay C, Stout RL (2006). Delusional versus nondelusional body dysmorphic disorder: clinical features and course of illness. J Psychiatr Res 40: 95–104.
Putnam MC, Steven MS, Doron KW, Riggall AC, Gazzaniga MS (2010). Cortical projection topography of the human splenium: hemispheric asymmetry and individual differences. J Cogn Neurosci 22: 1662–1669.
Rubinov M, Sporns O (2010). Complex network measures of brain connectivity: uses and interpretations. NeuroImage 52: 1059–1069.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 (Suppl 20): 22–33, quiz 34–57.
Shinoura N, Suzuki Y, Tsukada M, Katsuki S, Yamada R, Tabei Y et al (2007). Impairment of inferior longitudinal fasciculus plays a role in visual memory disturbance. Neurocase 13: 127–130.
Stam CJ, Reijneveld JC (2007). Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys 1: 3.
Stangier U, Adam-Schwebe S, Muller T, Wolter M (2008). Discrimination of facial appearance stimuli in body dysmorphic disorder. J Abnorm Psychol 117: 435–443.
van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE (2009). Efficiency of functional brain networks and intellectual performance. J Neurosci 29: 7619–7624.
Wang J, Wang L, Zang Y, Yang H, Tang H, Gong Q et al (2009). Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp 30: 1511–1523.
Watts DJ, Strogatz SH (1998). Collective dynamics of ‘small-world’ networks. Nature 393: 440–442.
Zalesky A, Fornito A, Harding IH, Cocchi L, Yucel M, Pantelis C et al (2010). Whole-brain anatomical networks: does the choice of nodes matter? NeuroImage 50: 970–983.
Zhang TJ, Wang JH, Yang YC, Wu QZ, Li B, Chen L et al (2011). Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J Psychiatr Neurosci 36: 23–31.
Acknowledgements
This work was supported by grants from the National Institute of Mental Health (K23 MH079212 and R01MH093535, Dr Feusner).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Results presented in part at the American College of Neuropsychopharmacology 50th Annual Meeting, Waikoloa, HA, USA, December 2011
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Arienzo, D., Leow, A., Brown, J. et al. Abnormal Brain Network Organization in Body Dysmorphic Disorder. Neuropsychopharmacol 38, 1130–1139 (2013). https://doi.org/10.1038/npp.2013.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.18
Keywords
This article is cited by
-
Visual training program for body dysmorphic disorder: protocol for a novel intervention pilot and feasibility trial
Pilot and Feasibility Studies (2018)
-
Neurocognitive Functioning in Young Adults with Subclinical Body Dysmorphic Disorder
Psychiatric Quarterly (2018)
-
Mental health: Monsters in the mirror
Nature (2015)
-
Focusing on flaws
Nature Reviews Neuroscience (2013)