Abstract
Methamphetamine affects the hippocampus, a brain region crucial for learning and memory, as well as relapse to drug seeking. Rats self-administered methamphetamine for 1 h twice weekly (intermittent-short-I-ShA), 1 h daily (limited-short-ShA), or 6 h daily (extended-long-LgA) for 22 sessions. After 22 sessions, rats from each access group were withdrawn from self-administration and underwent spatial memory (Y-maze) and working memory (T-maze) tests followed by extinction and reinstatement to methamphetamine seeking or received one intraperitoneal injection of 5-bromo-2′-deoxyuridine (BrdU) to label progenitors in the hippocampal subgranular zone (SGZ) during the synthesis phase. Two-hour-old and 28-day-old surviving BrdU-immunoreactive cells were quantified. I-ShA rats performed better on the Y-maze and had a greater number of 2-h-old SGZ BrdU cells than nondrug controls. LgA rats, but not ShA rats, performed worse on the Y- and T-maze and had a fewer number of 2-h-old SGZ BrdU cells than nondrug and I-ShA rats, suggesting that new hippocampal progenitors, decreased by methamphetamine, were correlated with impairment in the acquisition of new spatial cues. Analyses of addiction-related behaviors after withdrawal and extinction training revealed methamphetamine-primed reinstatement of methamphetamine-seeking behavior in all three groups (I-ShA, ShA, and LgA), and this effect was enhanced in LgA rats compared with I-ShA and ShA rats. Protracted withdrawal from self-administration enhanced the survival of SGZ BrdU cells, and methamphetamine seeking during protracted withdrawal enhanced Fos expression in the dentate gyrus and medial prefrontal cortex in LgA rats to a greater extent than in ShA and I-ShA rats. These results indicate that changes in the levels of the proliferation and survival of hippocampal neural progenitors and neuronal activation of hippocampal granule cells predict the effects of methamphetamine self-administration (limited vs extended access) on cognitive performance and relapse to drug seeking and may contribute to the impairments that perpetuate the addiction cycle.
Similar content being viewed by others
INTRODUCTION
The psychostimulant drug methamphetamine is highly addictive, can be acutely lethal, and can result in long-term neuroadaptive alterations with many implications for health and well-being. Converging lines of evidence from clinical and preclinical studies have revealed that methamphetamine dependence is linked to a decreased volume of limbic-related structures (Schwartz et al, 2010; Simon et al, 2010), altered hippocampal morphology (Mandyam et al, 2008; Kim et al, 2010), and hippocampal-related deficits in learning and memory (Rogers et al, 1999, 2008). Given that the hippocampus is essentially involved in learning and memory processes (Black et al, 1977; Morris et al, 1982; Eichenbaum et al, 1992; Riedel et al, 1999; Scoville and Milner, 2000; Burgess et al, 2002) and mounting evidence that suggests a role of an important form of hippocampal plasticity, namely adult hippocampal neurogenesis in hippocampus-dependent learning and memory (van Praag et al, 1999; Shors et al, 2001; Jessberger et al, 2009; for reviews, see Leuner et al, 2006; Kim et al, 2011), we wished to determine whether methamphetamine-induced inhibition of hippocampal neurogenesis contributes to methamphetamine-induced impairments in hippocampus-dependent cognitive behaviors.
With respect to addiction research, clinical and preclinical studies have revealed that addictive behaviors, including relapse to drug taking, are regulated by mesocorticolimbic neural circuitry, which includes a projection to the hippocampal formation (Nestler, 2001; Kalivas and McFarland, 2003; Koob and Volkow, 2010). With respect to methamphetamine addiction, a few reports have suggested that the hippocampus may contribute to specific aspects of methamphetamine addiction (Ursin et al, 1966; Ricoy and Martinez, 2009) and relapse (Tani et al, 2001; Hiranita et al, 2006; Shen et al, 2006; Rogers et al, 2008). Thus, it appears that plasticity in the hippocampus after chronic methamphetamine use may contribute to some of the addictive behaviors associated with chronic methamphetamine use.
Recent evidence demonstrated that methamphetamine exposure alters adult hippocampal neurogenesis (Hildebrandt et al, 1999; Teuchert-Noodt et al, 2000; Mandyam et al, 2008). Adult hippocampal neurogenesis is a spontaneous, multi-stage process that includes the birth, survival, and integration of granule cell neurons in the subgranular zone (SGZ) of the dentate gyrus of the adult hippocampus (Altman and Das, 1965). Methamphetamine alters every stage of hippocampal neurogenesis, and these effects vary by dose, duration, pattern of drug exposure, and timing of drug exposure before labeling the neural progenitors (Hildebrandt et al, 1999; Teuchert-Noodt et al, 2000; Mandyam et al, 2008; Yuan et al, 2011). Therefore, the inhibitory effect of methamphetamine on the dynamic regenerative capacity of the adult hippocampus is now being considered a precursor of methamphetamine-induced neurodegeneration in this region (Canales, 2007, 2010).
Altogether, significant advances are being made in understanding how methamphetamine impairs adult hippocampal neurogenesis. However, it is unknown whether methamphetamine-induced inhibition of hippocampal neurogenesis correlates with methamphetamine-induced impairment of hippocampus-dependent memory. In addition, given that the hippocampus has a role in methamphetamine-seeking behavior (Hiranita et al, 2006), unknown is how withdrawal from methamphetamine influences the survival of hippocampal progenitors, and such studies would be important to determine whether alterations in the survival of adult-born granule neurons contributes to the reinstatement of methamphetamine-seeking behavior. Importantly, the use of various methamphetamine exposure paradigms via self-administration provides the means to pursue such correlative studies, such as whether the pattern of methamphetamine intake could be attributable to methamphetamine's differential influence on hippocampus-dependent memory, relapse behavior, and spontaneous neuroplasticity.
We thus tested the hypothesis that a history of extended access vs limited access methamphetamine intake is associated with specific spatial and working memory impairments dependent on the hippocampus. To test this hypothesis, the integrity of spatial memory under low-incentive conditions and working memory under high cognitive demands and incentive conditions was assessed 3–15 days after the last self-administration session in rats that self-administered methamphetamine under a fixed-ratio schedule for 1 h twice weekly (intermittent-short-access [I-ShA]), 1 h daily (short-access [ShA]), or 6 h daily (long-access [LgA]; Cryan et al, 2003; George et al, 2008). We employed a spontaneous alternation procedure that used a Y-maze (ie, a task sensitive to hippocampal dysfunction; Conrad et al, 1996) and a delayed nonmatching-to-sample procedure that used a T-maze (ie, a task sensitive to hippocampal and prefrontal cortical dysfunction; Gerlai, 1998, 2001; Wall and Messier, 2001; Lalonde, 2002; George et al, 2008; Sudai et al, 2011). After testing for cognitive performance, the rats underwent extinction training and drug-seeking behavior testing (ie, tasks sensitive to nucleus accumbens [NAc] core, prefrontal cortex [PFC], and hippocampal dysfunction; Hiranita et al, 2006; Feltenstein and See, 2008; Noonan et al, 2010) to test the central hypothesis that varied methamphetamine access produces differential effects on memory and relapse tasks that depend on the hippocampus and that the behavioral outcomes correlate with changes in the levels of hippocampal neural progenitors. In the context of the above studies, a different cohort of I-ShA, ShA and LgA rats was injected with 5-bromo-2′-deoxyuridine (BrdU) to label proliferating synthesis (S)-phase neural progenitors and euthanized 2 h later to measure cell proliferation and 28 d later to measure cell survival.
MATERIALS AND METHODS
Animals
Seventy-six adult, male Wistar rats (Charles River), weighing 200–250 g at the start of the experiment, were housed two per cage in a temperature-controlled vivarium under a reverse light/dark cycle (lights off 0800–2000 hours). Food and water were available ad libitum. Fifty-four rats underwent surgery for catheter implantation for intravenous methamphetamine self-administration (Mandyam et al, 2007b). Methamphetamine hydrochloride (generously provided by the National Institute on Drug Abuse) was dissolved in physiological saline (0.9%), and methamphetamine self-administration was performed 5 days per week. For baseline training sessions, the rats were allowed to self-administer methamphetamine at a dose of 0.05 mg/kg per injection under an FR1 schedule for 8–10 sessions. After baseline training, the rats were divided into three methamphetamine access groups, balanced by the number of injections per session during the last three baseline sessions. The rats in each group were divided into Meth, Withdrawal, and Reinstatement groups balanced by their baseline performance. During the escalation period, one group of rats (LgA, n=18) was allowed to self-administer 0.05 mg/kg per injection of methamphetamine for 6 h per day under an FR1 schedule, whereas the other group (ShA, n=18 per group) was allowed to do so for 1 h per day under an FR1 schedule. A third group (I-ShA, n=18) was exposed to methamphetamine on Mondays and Thursdays (each week). All of the rats were 19–20 weeks old when perfused (Mandyam et al, 2008). All procedures were performed during the dark cycle.
Sixteen to 22 h after the last methamphetamine session, some rats from all three access groups received one injection of 150 mg/kg BrdU, intraperitoneal (Boehringer Mannheim Biochemica), dissolved in 0.9% saline and 0.007N NaOH at 20 mg/ml and survived for 2 h (Meth group; Figure 1a; n=5 for all methamphetamine groups; n=5 for drug-naive controls) or 28 days (Withdrawal group; Figure 1a; n=4–5 for all methamphetamine groups; n=5 for drug-naive controls), after which they were anesthetized with chloral hydrate and perfused transcardially as described previously (Mandyam et al, 2004). Thirty-seven rats (control, n=12; I-ShA, n=8; ShA, n=8; LgA, n=9) participated in the behavior studies. The rats were tested on the Y-maze and T-maze and for extinction behavior followed by methamphetamine-primed reinstatement (Reinstatement group; Figure 1a). The rats that underwent reinstatement were euthanized 1–2 h after the last session. Serial coronal 40 μm sections were obtained on a freezing microtome, and sections from the brain (−1.4 to −6.7 mm from bregma; Paxinos and Watson, 1997) were stored in 0.1% NaN3 in 1 × phosphate-buffered saline at 4°C.
Time-line of methamphetamine self-administration and daily intake of methamphetamine (mg/kg). (a) Schematic representation of experimental procedure. All rats self-administered methamphetamine in 1 h baseline sessions for 10 days (indicated by dashed lines for Monday–Friday (MTWTF)). Following baseline self-administration, the rats were divided into I-ShA, ShA, and LgA groups and self-administered methamphetamine for 22 sessions. Following 22 sessions of methamphetamine, the rats were subdivided into three groups: Reinstatement (behavior group that did not receive any BrdU injections and euthanized after 29 days after the last self-administration session; I-ShA, n=8; ShA, n=8; LgA, n=9); Meth (methamphetamine group injected with BrdU and euthanized 2 h after injection; I-ShA, n=5; ShA, n=5; LgA, n=5) and Withdrawal (protracted withdrawal group injected with BrdU at the same time as the Meth group and euthanized after 28 days of withdrawal; I-ShA, n=5; ShA, n=5; LgA, n=4). (b) Methamphetamine intake over 22 sessions in mg/kg in I-ShA, ShA, and LgA rats after baseline sessions. (c) Average daily (left axis) and total (right axis) methamphetamine intake (mg/kg) in I-ShA, ShA, and LgA rats. *p<0.05 in (b), compared with day 1 of escalation; Cp<0.05, compared with LgA in (c). I-ShA, n=18; ShA, n=18; LgA, n=18. Data are expressed as mean±SEM.
Spontaneous Alternation Behavior (Y-maze)
The Y-maze was performed in an automated apparatus connected to a camera and computer that monitored movement between the arms and time spent in each arm. Novelty was guided by distinct spatial cues. The experimenter was only responsible for placing the rat into the apparatus and removing the rat after the testing period. Care was taken to ensure that the rat was stress-free during testing. The Y-maze was constructed of black plastic with three arms (61 × 14 × 35 cm3) that extended from a central platform at a 120° angle (Figure 2a). Seventy-two hours after self-administration, each rat was placed in the middle of the maze and allowed to move freely in the three arms of the maze during a 10 min testing period. An arm entry was defined as the entry of four paws into one arm. The sequence of the arm entries was recorded using infrared beams. Alternation was defined as multiple entries into the three different arms on overlapping triplet sets. The percentage of spontaneous alternation was calculated as the ratio of the actual to possible alternations (defined as the total number of arm entries minus 2) multiplied by 100: alternation (%)=[(number of alternation)/(total arm entries-2)]/100 (Conrad et al, 1996). The Y-maze task was performed by an experimenter blind to the treatment groups.
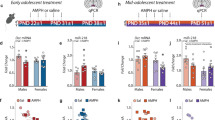
Effects of methamphetamine self-administration on spontaneous alternation behavior in the Y-maze and delayed non-matching-to-sample task in the T-maze. (a) Illustration of Y-maze task. (b) Illustration of T-maze task. (c) Percentage of spontaneous alternation in the Y-maze. #p<0.05, compared with 50% alternation (chance); ap<0.05, compared with I-ShA; *p<0.05, compared with naive. (d) Percentage of correct responses in the delayed non-matching-to-sample task. #p<0.05, compared with LgA; *p<0.05, compared with naïve; ap<0.05, compared with I-ShA; bp<0.05, compared with ShA. (e–g) Rule learning and solving strategies in Reinstatement rats. (e) Illustration of rule learning and delayed non-matching-to-sample task with 180° rotation and two different possible navigational strategies—egocentric and allocentric—used to solve the delayed non-matching-to-sample task. (f) Total number of days to reach the criterion in the delayed non-matching-to-sample task (ie, latency to achieve >80% alternation during training). (g) Expected and observed results after rotation of the T-maze. The left panel represents performance expected after the rotation if the three groups had used an egocentric or allocentric strategy, based on baseline performance with the 10 s delay. The right panel represents the observed results after the rotation. Notice that all of the groups used the same allocentric strategy. I-ShA, n=8; ShA, n=8; LgA, n=9. Data are expressed as mean±SEM. *p<0.05, compared with control (panels c, d, f); bp<0.05, compared with ShA (panels c, d); cp<0.05, compared with LgA (panels c, f).
Delayed Non-Matching-to-Sample Task (T-maze)
The T-maze procedure began 24 h after the Y-maze task and was performed as described previously (Figure 2b; George et al, 2008). Starting from Day 4 after the last self-administration session, the rats were food-deprived to 85% free-feeding body weight and habituated to the T-maze until they readily consumed a sucrose pellet placed at the end of each arm of the T-maze. To control for the nonspecific effects of drug self-administration on behavioral performance, age-matched drug-naive (ie, without a history of self-administration) rats were tested simultaneously. I-ShA, ShA, LgA, and control rats did not differ in body weight before or during the food-deprivation period. Rats from all groups were run in parallel, in a random order, and the experimenters were blind to the group. After habituation, the rats were trained for 10 trials/session on a non-matching-to-sample task. Each trial consisted of one forced-choice run and one free-choice run (rewarded only if the rat entered the opposite arm). After reaching the acquisition criterion (>70% correct responses during 2 consecutive days), a variable delay (10 and 130 s) was introduced between the forced and free runs, and the number of trials per session was increased to 16. The first trial consisted of a 10 s delay followed by 15 trials with randomly chosen delays (10 and 130 s). The delay sequences were chosen to avoid repetition of the same delay or the same sequence and with an equal representation of each delay. The rats were tested in this protocol for 2–3 sessions that corresponded to 15–17 days after the last self-administration session. The intertrial interval (ie, the time between the second run of trial n and the first run of trial n+1) was fixed at 120 s to avoid proactive interference. The number of trials necessary to reach the criterion and percentage of correct responses were calculated.
Extinction Testing
After 19 days of abstinence from self-administration, the rats underwent daily 1 h extinction sessions. During the extinction sessions, the rats were not connected to the infusion apparatus. Responses on either the active or inactive lever were recorded and did not result in programmed consequences (ie, no infusions and no conditioned stimulus presentations). Extinction training consisted of a minimum of 10 sessions plus additional extinction training sessions as needed until the rats reached the extinction criterion (<20 active lever presses per session for two consecutive sessions).
Reinstatement Testing
Seventy-two hours after the final extinction session, the rats from all of the methamphetamine groups were placed into the methamphetamine-paired context (ie, the same operant box used for self-administration sessions) for 1 h, during which they were connected to the infusion apparatus to allow for a similar interaction with the spatial elements of the context as during methamphetamine self-administration training. Lever presses were used as a measure of drug seeking, and responses on either the active or inactive lever were recorded and did not result in an infusion of fluids through the catheter or other programmed consequences (ie, conditioned stimulus presentations). After the first hour in the self-administration chamber, the rats received an intraperitoneal injection of 0, 0.5, and 1 mg/kg methamphetamine on day 1, 2, or 3 in a counterbalanced order and were put back in the same self-administration chamber after 30 min for an additional hour. Active lever responses before and after methamphetamine priming were analyzed, and methamphetamine-primed reinstatement is expressed as the total number of lever presses per animal per dose of methamphetamine after priming. All of the rats were euthanized 1–3 h post reinstatement testing. The rats that were euthanized within 90 min post testing were used for fluorescent (Fos) analysis.
Antibodies, Immunohistochemistry, Microscopic Analysis, and Quantification
The following primary antibodies were used for immunohistochemistry (IHC): Ki-67 (1 : 1000), BrdU (1 : 400), activated caspase 3 (AC-3; 1 : 500), and c-Fos (1 : 1000). The left and right hemispheres of every twelfth (BrdU, Ki-67, AC-3) section through the rat hippocampus were slide-mounted, coded, and dried overnight before IHC. For Fos IHC, six bilateral sections that contained the dentate gyrus, two bilateral sections that contained the mPFC (including the anterior cingulate, prelimbic, and infralimbic cortices), and two bilateral sections that contains the NAc core were used for cell quantification from each rat from each group. The sections were pretreated (Mandyam et al, 2004), blocked, and incubated with the primary antibodies (BrdU, Ki-67, AC3, Fos) followed by biotin-tagged secondary antibodies. Immunoreactive cells in the SGZ (ie, cells that touched and were within three cell widths inside and outside the hippocampal granule cell-hilus border for 2 h BrdU, Ki-67) or granule cell layer (GCL; 28 day BrdU, AC-3, and Fos) were quantified with a Zeiss Axiophot photomicroscope ( × 400 magnification) using the optical fractionator method, in which sections through the dentate gyrus (−1.4 to −6.7 mm from bregma; Paxinos and Watson, 1997) were examined. Cells in the SGZ and GCL were summed and multiplied by 12 to give the total number of cells (Eisch et al, 2000). Fos cells in the mPFC (3.7 and 3.2 mm from bregma) and NAc core (1.2 and 1.0 mm from bregma) were examined, and cells from the left and right hemispheres that were localized in the counting frame (mPFC, 0.09 mm2; NAc, 0.078 mm2) were visually quantified. The total number of immunoreactive cells both in the left and right hemispheres was counted and are expressed as the total number of immunoreactive cells per mm2 in each rat averaged across rats within a group.
Data Analysis
The methamphetamine self-administration data are expressed as the mean mg/kg per session of methamphetamine self-administration for each group of rats. The effect of the session duration on methamphetamine self-administration per session and during the first hour of a session was examined over the 22 escalation sessions using a two-way repeated-measures analysis of variance (ANOVA; session duration × daily session; SPSS software) followed by the Student–Newman–Keuls post hoc test. The pattern of responding for methamphetamine is expressed as the mean mg/kg per hour over 6 h sessions in LgA rats and were compared between the first and >10th escalation sessions. Differences in the rate of responding between the first and other escalation sessions were evaluated using the paired t-test. For the Y-maze study, the four groups (naive, I-ShA, ShA, and LgA) were used as between-subjects factors, and novelty-induced alternation in the Y-maze or self-administration sessions was used as the within-subjects factor. For the T-maze study, the four groups (naive, I-ShA, ShA, and LgA) were used as between-subjects factors, and the two delays (10 and 130 s) in the T-maze or self-administration sessions were used as the within-subjects factors. ANOVA was used to compare the Y-maze and T-maze performance with chance performance. Lever responding during extinction and during the reinstatement tests was also assessed by ANOVA. For the BrdU, Ki-67, AC-3, and Fos analyses, one- or two-way ANOVA was used when appropriate. Nonlinear regression analyses were performed using Pearson's correlation tests. The data are expressed as mean±SEM in all graphs.
RESULTS
Escalation in Methamphetamine Intake is Evident in LgA Rats
Total and daily methamphetamine intake was significantly different between the methamphetamine groups (Figures 1b and c; average intake over 22 sessions: F2, 54=22.7, p<0.0001), and the post hoc analysis revealed significantly higher intake in LgA rats compared with I-ShA and ShA rats (all p<0.001). Methamphetamine self-administration during the first hour was considerably higher in LgA vs ShA and I-ShA rats (F2, 54=7.4, p<0.05) following session 10 and was maintained until session 22 (p<0.05).
Methamphetamine Self-Administration Improves Spatial Memory in I-ShA Rats and Impairs Spatial and Working Memory in LgA Rats
To test spatial memory under low-incentive conditions, rats from the control, I-ShA, ShA, and LgA rats (reinstatement group) were tested in a novelty-induced alternation task in the Y-maze (Figure 2a). Methamphetamine rats and controls exhibited a higher percentage of alternation behavior compared with chance (Figure 2c; F4, 45=13.5, p<0.001), demonstrating the incentive value of novelty. The post hoc analysis revealed a significantly higher response in control (p<0.001), I-ShA (p<0.001), and ShA (p=0.027) rats compared with chance performance. LgA rats did not exhibit higher alternation compared with chance performance (p=0.454). Control, ShA, and LgA rats exhibited lower alternation compared with I-ShA rats (all p<0.05). LgA rats exhibited lower alternation compared with control and I-ShA rats (all p=0.001), suggesting that LgA rats were impaired under low-incentive conditions. In the delayed non-matching-to-sample task (Figure 2b), the multivariate analysis revealed a Delay × Group interaction (Figure 2d; F3, 33=10.9, p<0.001). I-ShA and ShA rats exhibited normal working memory performance compared with control rats when a short delay (10 s) between the acquisition trials and test trials was used. LgA rats had significantly poorer working memory performance compared with control, I-ShA, and ShA rats during the short delay (10 s) tests (all p<0.001). All of the rats performed equally well during the tests that involved a longer delay (130 s) between the acquisition trials and test trials.
Impaired Spatial and Working Memory in LgA Rats is not Attributable to Alterations in General Cognitive Ability
As I-ShA rats demonstrated an improvement in performance in the Y-maze task, we next determined whether these behavioral outcomes could be attributable to improved learning capability or improved spatial recognition in these rats (Figure 2e). I-ShA rats demonstrated a reduced latency to achieve greater than 80% alternation during the training phase of the T-maze task (Figure 2f; F3, 28=3.5, p=0.03) compared with control (p=0.04) and LgA (p=0.004) rats, suggesting improved learning capability. However, impaired performance on the Y-maze and T-maze in LgA rats under low-incentive and low working memory load (Figures 2c and d) was not associated with an impaired rate of learning (Figure 2f), suggesting preserved general cognitive function necessary to perform the task, such as sensation, perception, rule learning, behavioral selection, and action–outcome association. An alternative explanation for the decreased spatial and working memory performance in LgA rats is that they used a different navigational strategy to solve the Y-maze and T-maze tasks. Egocentric strategies (ie, orientation in space using proprioceptive information or intra-maze cues) and allocentric strategies (ie, orientation in space using extra-maze cues in the environment; Figure 2e; King and Corwin, 1992; Holscher and Schmidt, 1994; Nieto-Escamez et al, 2002) were tested. The effect of a 180° rotation of the T-maze between the acquisition and test trials was evaluated on working memory performance using a 10 s delay. The rotation shifted the position of the proximal cues in the T-maze by 180° compared with the distal cues in the room, the orientation of which were left unchanged (Figure 2e). In this case, an allocentric strategy (ie, the use of distal cues) would lead to good performance (now defined as an entrance into the same arm), whereas an egocentric strategy (ie, the use of intra-maze cues and proprioceptive information) would lead to poor performance (Figure 2e). After the rotation, all of the groups exhibited more than 90% correct responses in accordance with an allocentric strategy (Figure 2g; F3, 32=10.5, p<0.001), ruling out the hypothesis that a differential navigational strategy could explain the spatial and working memory impairments observed in LgA rats.
Methamphetamine Self-Administration Increases S-phase Cells in I-ShA Rats and Decreases S-phase Cells in LgA Rats in the Hippocampal Dentate Gyrus
Cell proliferation experiments determined whether enhanced/impaired performance on the Y-maze correlated with increases or decreases in the number of neural progenitors in the SGZ of the dentate gyrus. After 16-22 h the methamphetamine session I-ShA, ShA, LgA, and drug-naive control rats from meth group were injected with BrdU and euthanized 2 h later to label S-phase cells (Figure 1a). BrdU IHC revealed a normal pattern of S-phase cells in the dentate gyrus, with BrdU cells in the SGZ as reported previously (Mandyam et al, 2007a; Noonan et al, 2008). BrdU cells at this proliferation time point appeared small, irregular, and in clusters in the control and all methamphetamine groups (Figure 3a). Cell quantification of S-phase BrdU-positive cells demonstrated that methamphetamine significantly altered the number of BrdU cells (Figure 3b; F3, 19=11.5, p<0.001). Specifically, I-ShA rats exhibited an increased number of BrdU cells compared with controls and the other methamphetamine groups (all p<0.05), and LgA rats had reduced BrdU cells compared with controls and I-ShA rats (all p<0.05).
Effects of methamphetamine self-administration on cell proliferation. (a) Photomicrograph of proliferating cells in the SGZ of the hippocampal dentate gyrus labeled with a BrdU antibody. BrdU-labeled 2-h-old proliferating cells are visible as irregularly shaped cells in a cluster. GCL, granule cell layer; H, hilus. Scale bar in (a)=20 μm. (b) Quantitative analysis of BrdU cell counts from Meth rats from serial coronal sections. Total number of BrdU cells per hippocampus from each access group. Control, n=5; I-ShA, n=5; ShA, n=5; LgA, n=5. Data are expressed as mean±SEM; *p<0.05, compared with control; ap<0.05, compared with I-ShA; bp<0.05, compared with ShA; cp<0.05, compared with LgA. (c) Linear regression analysis of methamphetamine self-administration-induced changes in proliferation and cognitive behavior. Total number of 2 h BrdU-labeled cells per hippocampus per animal (gray circles, left axis) and percent spontaneous alternation per animal (black circles, right axis) plotted against total methamphetamine consumed during 22 self-administration sessions. I-ShA, n=5–8; ShA, n=5–8; LgA, n=5–9.
Total Amount of Methamphetamine Self-Administered Negatively Correlates with Cell Proliferation in the Dentate Gyrus and Spontaneous Alternation on the Y-maze
As the extent of alternation on the Y-maze and cellular proliferation in the dentate gyrus varied across rats in the methamphetamine groups, the percentage of alternation on the Y-maze and 2 h old BrdU cells were compared with the total amount of methamphetamine intake over 22 sessions in the methamphetamine rats using linear regression analysis. There was a significant correlation between performance on the Y-maze task and amount of methamphetamine consumed (p<0.001, r=0.43) and cell proliferation and the amount of methamphetamine consumed (p=0.02, r=0.35) when all of the methamphetamine rats were combined (Figure 3c).
Protracted Withdrawal from Methamphetamine Self-Administration Produces Methamphetamine-Primed Reinstatement of Methamphetamine-Seeking Behavior in I-ShA, ShA, and LgA Rats, and this Behavior is Enhanced in LgA Rats
Following the cognitive tests, all of the methamphetamine rats in the reinstatement group underwent several extinction sessions in their operant chambers. During the extinction sessions, methamphetamine was no longer available, and drug-paired lever responding readily declined across daily extinction sessions (Figure 4a). The extinction session duration was maintained at 1 h for all of the groups, and responses during the sessions gradually declined to the criterion of ⩽20 lever presses (significant main effect of Day, F18, 172=7.7, p<0.001), which was reached with 6–10 days of training (Figure 4a; p<0.05 vs day 1). All rats extinguished before reinstatement testing.
Extinction and reinstatement of methamphetamine seeking. (a) Decreased lever responding on the previously methamphetamine-paired lever in daily 1 h extinction trials. No significant differences were seen across access conditions. The extinction criterion was reached after day 6 in all groups (*p<0.05, compared with day 1 of extinction responding). (b) Methamphetamine-primed reinstatement expressed as active lever presses after methamphetamine priming. *p<0.05, compared with saline responding; ap<0.05, compared with I-ShA rats; bp<0.05 compared with ShA rats). I-ShA, n=8; ShA, n=8; LgA, n=9. Data are expressed as mean±SEM.
All methamphetamine rats showed significant methamphetamine-primed reinstatement (Figure 4b; F4, 45=14.4, p<0.001). A significant difference between methamphetamine groups was seen during methamphetamine-primed reinstatement (Figure 4b; F4, 45=4.8, p=0.01). I-ShA, ShA, and LgA rats showed significant methamphetamine-primed reinstatement after 0.5 mg/kg methamphetamine priming (all p<0.05). After the 1.0 mg/kg dose of methamphetamine, LgA rats demonstrated greater reinstatement than I-ShA and ShA rats (all p<0.05).
Protracted Withdrawal from Methamphetamine Self-Administration Increases the Proliferation and Survival of Progenitors in LgA Rats in the Dentate Gyrus of the Hippocampus
As methamphetamine increased S-phase BrdU cells in I-ShA rats and decreased S-phase BrdU cells in LgA rats (Figure 3b), we then examined whether protracted withdrawal from methamphetamine self-administration for 4 weeks would normalize SGZ proliferation and cell survival in I-ShA, ShA, and LgA rats. BrdU was given to the withdrawal group at the same time as the meth group, whereas meth group rats were killed 2 h later, withdrawal group rats were killed 4 weeks later, corresponding to the same time frame when reinstatement group rats were euthanized (Figure 1a). Thus, BrdU cells in the withdrawal group represented surviving, not proliferating, S-phase cells. Mature BrdU cells in withdrawal group rats were round and less clustered and presented more punctate BrdU staining (Figure 5a), typical of SGZ cells 4 weeks after BrdU injection (Cameron and McKay, 2001; Dayer et al, 2003). As BrdU proliferating cells increased in I-ShA rats and decreased in LgA rats compared with controls (Figure 3b), a higher number of mature BrdU cells in I-ShA rats and a lower number of mature BrdU cells in LgA rats were expected after withdrawal. Protracted withdrawal increased the number of mature BrdU cells (Figure 5c; F3, 18=3.4, p=0.05), with LgA rats having more BrdU cells compared with I-ShA rats (p=0.03). An explanation for the surprisingly normal level of mature BrdU cells in I-ShA and LgA rats (Figure 5c) after protracted withdrawal could be the altered rate of survival of BrdU S-phase cells. To test this possibility, and because BrdU was injected on the same day in rats in the meth and withdrawal groups (Figure 1a), the ratio of S-phase BrdU cells to mature BrdU cells was calculated in all rats. Methamphetamine self-administration had a significant effect on the rate of survival of BrdU progenitors (Figure 5d; F3, 18=9.8, p=0.001), with LgA rats having more cells that survived compared with control (p=0.001), I-ShA (p<0.001), and ShA (p=0.005) rats. ShA rats also exhibited enhanced cell survival compared with I-ShA rats (p=0.046).
Effects of protracted withdrawal from methamphetamine self-administration and methamphetamine-primed reinstatement on proliferation, survival, and neuronal activation in the dentate gyrus, mPFC, and NAc core. (a) Photomicrograph of mature 28-day-old BrdU cells in the GCL of the hippocampal dentate gyrus. Mature BrdU cells are round to oval in shape, resembling surrounding granule cell neurons. (b) Photomicrograph of proliferating Ki-67 cells in the SGZ of the hippocampal dentate gyrus. Ki-67 cells are irregular and often found in clusters of cells that resemble the immature morphology of proliferating progenitors. GCL, granule cell layer; H, hilus. Scale bar=15 μm in (a) and 30 μm in (b). (c) Quantitative analysis of total number of 28-day-old BrdU cells in the dentate gyrus; ap<0.05, compared with I-ShA. (d) Proportion of 2 h BrdU cells (meth group) that survived to become BrdU neurons (withdrawal group) in all access groups. The rate of survival was calculated as the ratio of withdrawal group BrdU cell counts to meth group BrdU cell counts. *p<0.05, compared with control; ap<0.05, compared with I-ShA; bp<0.05, compared with ShA; cp<0.05, compared with LgA. (e) Quantitative analysis of total number of Ki-67 cells in the dentate gyrus from withdrawal group rats and reinstatement group rats. *p<0.05, compared with controls. (f–h) Quantitative analysis of total number of Fos-labeled cells in the GCL of the dentate gyrus (f), mPFC (g), and NAc core (h). *p<0.05, compared with controls; ap<0.05, compared with I-ShA; bp<0.05, compared with ShA; #p<0.05, compared with withdrawal rats from the same access group. Control, n=5–13; I-ShA, n=5–8; ShA, n=5–8; LgA, n=4–9. Data are expressed as mean±SEM.
To examine whether protracted withdrawal altered the proliferation of SGZ cells, actively dividing SGZ cells in the withdrawal group were detected with an antibody against Ki-67, an endogenous marker of proliferation that is used interchangeably with short timeframe BrdU labeling to detect proliferating cells (Kee et al, 2002). Ki-67 cells were irregularly shaped and showed immature morphology that was consistent with proliferating cells and were often clustered (Figure 5b; Mandyam et al, 2008). Protracted withdrawal increased the number of Ki-67 cells in all of the methamphetamine groups, with a significant effect of drug on cell numbers (Figure 5e; F3, 18=3.7, p=0.05). Control rats had fewer Ki-67 cells compared with I-ShA (p=0.01), ShA (p=0.02), and LgA (p=0.05) rats. Cell death increased after withdrawal, reflected by cell counts for AC-3 cells (F3, 21=4.3, p=0.02), with LgA and I-ShA rats having more AC-3 cells compared with controls (all p<0.05; controls, 72±19; I-ShA, 265±124; ShA, 90±20; LgA, 319±143).
Methamphetamine Priming-Induced Reinstatement of Methamphetamine Seeking Enhances Neuronal Activation of Granule Cell Neurons in LgA Rats without Effecting Proliferation of SGZ Progenitors
After demonstrating the distinct effects of methamphetamine self-administration vs protracted withdrawal on the number of proliferating cells and adult-generated mature BrdU cells, we then explored whether reinstatement of methamphetamine seeking during protracted withdrawal differentially affects proliferation. Reinstatement group rats from all of the methamphetamine groups learned to extinguish operant responses and were subsequently tested for methamphetamine-primed reinstatement. Because these rats were not injected with BrdU, proliferating cells were quantified by Ki-67 labeling (Figure 5b). Reinstatement of methamphetamine seeking normalized the number of Ki-67 cells that were increased during protracted withdrawal (Figure 5e; all p>0.05).
Methamphetamine-primed reinstatement produced a significantly greater number of Fos-positive cells in the GCL of the dentate gyrus compared with protracted withdrawal (Figure 5f; F6, 37=2.9, p=0.02). Specifically, reinstatement group LgA rats had a greater number of Fos-positive cells compared with controls (p=0.007) and withdrawal group LgA rats (p=0.001). Reinstatement group I-ShA and ShA rats did not exhibit significant changes in Fos cells compared with controls and withdrawal group I-ShA and ShA rats.
Methamphetamine Priming-Induced Reinstatement of Methamphetamine Seeking Enhances Neuronal Activation in the mPFC in LgA Rats
Methamphetamine-primed reinstatement produced a significantly greater number of Fos-positive cells in the mPFC compared with protracted withdrawal (Figure 5g; F6, 39=5.5, p<0.05). Specifically, reinstatement group LgA rats had a greater number of Fos-positive cells compared with control (p<0.005), I-ShA (p=0.001), ShA (p<0.001), and withdrawal group LgA rats (p=0.002). Reinstatement group I-ShA and ShA rats did not exhibit significant changes in Fos cells compared with controls and the withdrawal group I-ShA and ShA rats. No significant differences in Fos cell counts were seen in the NAc core region (Figure 5h).
Protracted Withdrawal-Induced Enhancement of Mature BrdU Cells in the Dentate Gyrus and Active Lever Responses during Reinstatement of Methamphetamine Seeking Positively Correlate with Total Amount of Methamphetamine Consumed
As the extent of methamphetamine-primed reinstatement and mature BrdU cells in the dentate gyrus varied across methamphetamine rats in the reinstatement and withdrawal groups, active lever presses during reinstatement testing and 28-day-old BrdU cells were compared with the total amount of methamphetamine intake over 22 sessions using linear regression analysis. There was a significant correlation between active lever responses during methamphetamine-primed reinstatement and the amount of methamphetamine consumed (p<0.001, r=0.54) and between mature BrdU cells and the amount of methamphetamine consumed (p<0.001, r=0.64) when all of the methamphetamine rats were combined (Figure 6a).
Linear regression analysis. (a) Protracted withdrawal from methamphetamine self-administration-induced changes in cell survival and methamphetamine-primed reinstatement. Total number of 28-day-old BrdU-labeled cells per dentate gyrus per rat (gray filled circles, left axis) and difference in active lever presses (saline vs 0.1 mg/kg methamphetamine priming) during reinstatement testing (black filled circles, right axis) plotted against total methamphetamine consumed during 22 self-administration sessions. I-ShA, n=5–8; ShA, n=5–8; LgA, n=5–9. (b) Total number of Fos-labeled cells per dentate gyrus (black filled circles, left axis), mPFC (white filled circles, right axis), and NAc core (gray filled circles, right axis) per rat plotted against difference in active lever presses (saline vs 0.1 mg/kg methamphetamine priming) during reinstatement testing 60–90 min before euthanasia. I-ShA, n=5; ShA, n=5; LgA, n=4.
Fos Expression in the Dentate Gyrus and mPFC Positively Correlates with Active Lever Responses during Reinstatement to Methamphetamine Seeking
As the extent of methamphetamine-primed reinstatement varied across methamphetamine rats in the reinstatement group, active lever presses during reinstatement testing were compared with the number of Fos-labeled neurons in the GCL, mPFC, and NAc core in the methamphetamine rats using linear regression analysis. There was a significant correlation between active lever presses and the number of Fos-labeled granule neurons (p=0.005, r=0.65) and mPFC neurons (p=0.008, r=0.62) when all of the methamphetamine rats were combined (Figure 6b).
DISCUSSION
The present results highlight how varied access to methamphetamine self-administration alters memory and cognitive performance dependent on the hippocampus and cellular proliferation in the dentate gyrus of the hippocampus. Regression analysis of methamphetamine intake and behavioral performance and cellular proliferation demonstrated that alterations in spontaneous plasticity maintained by adult neural stem cells can predict cognitive performance dependent on the hippocampus as a function of total methamphetamine consumed. Withdrawal from methamphetamine self-administration enhanced methamphetamine seeking following priming in LgA rats compared with ShA and I-ShA rats, suggesting that the magnitude of methamphetamine seeking following priming is directly related to the amount of intake during prolonged methamphetamine self-administration (Yan et al, 2007; Rogers et al, 2008). Enhanced methamphetamine seeking following withdrawal correlated with increases in the survival of hippocampal neural progenitors and the neuronal activation of hippocampal granule cell neurons, suggesting that adult hippocampal plasticity may impact hippocampal function in general and relapse in particular (Noonan et al, 2008; Garcia-Fuster et al, 2011).
Intermittent and daily access to methamphetamine self-administration was performed to mimic the intermittently low and continued high levels of methamphetamine during recreational use in humans that may distinctly affect memory and cognitive function. With regard to psychomotor/cognitive performance in humans, there is general consensus about enhanced cognitive performance with acute (recreational) methamphetamines in methamphetamine-exposed individuals (Johnson et al, 2000, 2005; Hart et al, 2003; Silber et al, 2006) and deficits in cognitive performance in methamphetamine addicts (Scott et al, 2007; Parrott et al, 2011). The current preclinical findings of enhanced spatial memory in the Y-maze and learning in the T-maze by I-ShA rats and impaired performance in LgA rats, therefore, generally agree with the clinical results (Rogers et al, 2008; Reichel et al, 2011). Although merely speculative, our finding of cognitive performance in I-ShA, ShA, and LgA rats supports the hypothesis that the reinforcing effects of stimulants are increased when performance is perceived to be improved following drug administration (Silverman et al, 1994; Comer et al, 1997; Jones et al, 2001; Stoops et al, 2005), enhancing drug intake with increased access to the drug and mimicking the ‘loss of control’ over drug use observed in human addicts (Koob et al, 2004; Morgan and Roberts, 2004).
As varied access to methamphetamine distinctly altered hippocampus-dependent memory and because hippocampal neurogenesis within the hippocampal formation was proposed to contribute to the acquisition and retention of certain hippocampus-dependent memories (Leuner et al, 2006), a different cohort of methamphetamine rats was injected with BrdU to determine whether hippocampus-dependent behavioral outcomes correlated with changes in the levels of hippocampal neural progenitors. We previously found increased neural progenitors and immature neurons in the dentate gyrus in I-ShA rats, even after 42 sessions of self-administration (Mandyam et al, 2008). We hypothesized that the increases in the number of neural progenitors would be evident at an earlier time point that coincided with the time point that analyzed behavioral performance. Our results demonstrate that I-ShA rats have an increased number and LgA rats have a decreased number of BrdU progenitors in the dentate gyrus, and methamphetamine-induced alterations in BrdU progenitors correlate with methamphetamine-induced alterations in hippocampus-dependent memory (Figure 3c). These results, therefore, highlight the novel role of drug-induced adult neuroplasticity in cognitive function. Although care must be taken in comparing correlations of dentate gyrus progenitors and cognitive performance from drug self-administration vs non-drug studies (van Praag et al, 1999; Santarelli et al, 2003), our findings may provide insights into some important functions of spontaneous hippocampal plasticity that were previously underappreciated. Nevertheless, I-ShA methamphetamine-induced new neurons may transiently participate in memory storage and eventually outlive their usefulness for cognitive performance, perhaps becoming important for some other functions, including methamphetamine-seeking behavior.
The general pattern of extinction responding after withdrawal from methamphetamine self-administration was similar in all methamphetamine groups (Figure 4a). However, methamphetamine priming increased methamphetamine seeking, the magnitude of which was proportional to the amount of methamphetamine intake (Yan et al, 2007; Rogers et al, 2008). The analysis of mature BrdU cells and proliferating Ki-67 cells in the dentate gyrus in rats in the withdrawal groups addressed the possibility that the increased methamphetamine-primed reinstatement in LgA rats was likely related to differences in persistent neuroplastic effects produced during protracted withdrawal after LgA methamphetamine (Noonan et al, 2008; Garcia-Fuster et al, 2011). As I-ShA methamphetamine increased and LgA methamphetamine decreased BrdU progenitors, we hypothesized that protracted withdrawal would normalize the effects on proliferation and would not affect the survival of progenitors in all groups. Notably, protracted withdrawal maintained the enhanced proliferation in I-ShA rats, reflected by increased Ki-67 cell numbers. Enhanced proliferation during self-administration and withdrawal in I-ShA rats did not produce an increase in mature BrdU cells. Importantly, the lower number of mature BrdU cells was attributable to a reduced rate of BrdU survival in I-ShA rats, suggesting withdrawal-specific effects on later stages of neuronal development, including the maturation and differentiation of granule neurons (Noonan et al, 2008; Garcia-Fuster et al, 2011).
In LgA rats, protracted withdrawal increased proliferation, reflected by increased Ki-67 cells. The enhanced proliferation could be attributable to a compensatory effect (ie, decreased cell death) that might have occurred for the decreased population of progenitors (2 h BrdU cells) during self-administration. The analysis of AC-3 cells rules out this possibility because protracted withdrawal increased apoptosis in LgA rats. Alternatively, the modulation of the cell division of progenitors, such as the increased rate of symmetric division, could be responsible for the enhanced number of progenitors during protracted withdrawal (Noonan et al, 2008) and therefore the normal number of mature BrdU cells in LgA withdrawal group rats. As proliferation was not measured in LgA rats during early withdrawal and mature BrdU cells mostly reflect the survival of progenitors born during BrdU injection, an alternative explanation for the normal number of mature BrdU cells in LgA rats could be compensatory changes that occur in the survival rate of progenitors born during BrdU injection. The ratio of BrdU progenitors to mature BrdU cells demonstrates that protracted withdrawal after LgA methamphetamine increased the rate of survival of BrdU progenitors (Figure 5c and d). This withdrawal-specific effect in LgA rats is worth noting because this maladaptive change could function to return the homeostasis of granule cell neuron turnover, or incubate drug, and drug-context associations relating to drug seeking (Vorel et al, 2001; Hiranita et al, 2006; Rademacher et al, 2006; Shen et al, 2006; Zhou and Zhu, 2006; Lasseter et al, 2010; Noonan et al, 2010; Luo et al, 2011). However, further functional exploration of this putative enhanced survival is warranted, as well as identification of other neuroadaptations during protracted withdrawal that may regulate the hippocampus and hippocampus-dependent relapse behavior (Tani et al, 2001; Hiranita et al, 2006; Zhou and Zhu, 2006; Lasseter et al, 2010; Noonan et al, 2010; Garcia-Fuster et al, 2011).
We next determined whether relapse behaviors following noncontingent exposure to methamphetamine produces changes in dentate gyrus neural progenitors and whether these changes are altered compared with protracted withdrawal-induced changes. Methamphetamine seeking normalized Ki-67-proliferating progenitors in all methamphetamine groups (Figure 5e). The normalization of the protracted withdrawal-induced enhancement of Ki-67 can be hypothesized to be an immediate effect of methamphetamine priming because the regulation of Ki-67 is immediate following any exposure to a novel stimuli (Kee et al, 2002; Brown et al, 2003). It is also possible that increases in proliferation and survival in LgA rats were withdrawal-specific, and these changes might be altered in reinstated rats that experience learning and methamphetamine-seeking behavior during protracted withdrawal (Gould et al, 1999; Neisewander et al, 2000). Therefore, one minor limitation of the present results is the multiple test design. Inevitably, the fact that cellular analysis was conducted in the reinstatement group of rats that performed multiple behavioral tests (ie, cognitive followed by relapse) suggests that some caution is warranted.
Lastly, we examined whether methamphetamine-primed reinstatement of methamphetamine seeking differentially regulates neuronal activation in methamphetamine groups with varied access in key brain regions that encompass the mesocorticolimbic dopamine system and compared the changes with protracted withdrawal. The mPFC, NAc core, and hippocampal regions were analyzed based on research from methamphetamine-seeking studies that demonstrated mechanistic and correlative evidence of the involvement of these brain regions in methamphetamine relapse (Hiranita et al, 2006, 2008). Specifically, the hippocampal dentate gyrus was also examined (Noonan et al, 2008, 2010; Garcia-Fuster et al, 2011). We determined whether Fos expression was altered because Fos, a product of the immediate early gene c-fos, is transiently induced by cocaine and amphetamines (Ruskin and Marshall, 1994; Neisewander et al, 2000; Ciccocioppo et al, 2001; Gross and Marshall, 2009). The present study demonstrated increases in Fos protein expression by methamphetamine-primed seeking in the mPFC and dentate gyrus, and these increases were related to methamphetamine-seeking behavior (Figure 6b). However, the present study failed to observe changes in Fos protein expression in the NAc core, supporting some studies that also did not observe changes in NAc Fos levels after exposure to cocaine-paired stimuli (Brown et al, 1992; Crawford et al, 1995). For example, previous studies reported that increases in Fos protein expression in the NAc after acute drug administration exhibit tolerance after repeated administration (Graybiel et al, 1990; Brown et al, 1992; Hope et al, 1992; Moratalla et al, 1996; Neisewander et al, 2000), suggesting that discrepancies in Fos expression may be attributable to differences in the amount or schedule of drug experience, drug dose, length of withdrawal period with or without drug-seeking behavior before testing, and anatomical subregion analyzed.
Altogether, the present study defined the novel and distinct impact of varied methamphetamine self-administration access and subsequent withdrawal and methamphetamine-seeking behavior during withdrawal on neural proliferation, survival, and neuronal activation of granule cell neurons. Future studies should seek to clearly understand intermittent vs extended access methamphetamine-induced alterations in the hippocampal neurogenic microenvironment. These studies may point to a critical and mechanistic role of the dentate gyrus neurogenesis in drug-induced memory impairment, drug craving, and ultimately relapse, perhaps via selective facilitation of recall of drug-context associations that stimulate relapse behaviors at the expense of recall of non drug memories.
References
Altman J, Das GD (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–335.
Black AH, Nadel L, O’Keefe J (1977). Hippocampal function in avoidance learning and punishment. Psychol Bull 84: 1107–1129.
Brown EE, Robertson GS, Fibiger HC (1992). Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci 12: 4112–4121.
Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003). Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467: 1–10.
Burgess N, Maguire EA, O’Keefe J (2002). The human hippocampus and spatial and episodic memory. Neuron 35: 625–641.
Cameron HA, McKay RD (2001). Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435: 406–417.
Canales JJ (2007). Adult neurogenesis and the memories of drug addiction. Eur Arch Psychiatry Clin Neurosci 257: 261–270.
Canales JJ (2010). Comparative neuroscience of stimulant-induced memory dysfunction: role for neurogenesis in the adult hippocampus. Behav Pharmacol 21: 379–393.
Ciccocioppo R, Sanna PP, Weiss F (2001). Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA 98: 1976–1981.
Comer SD, Collins ED, Fischman MW (1997). Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol 8: 677–690.
Conrad CD, Galea LA, Kuroda Y, McEwen BS (1996). Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci 110: 1321–1334.
Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP (1995). The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology (Berl) 120: 392–399.
Cryan JF, Hoyer D, Markou A (2003). Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry 54: 49–58.
Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA (2003). Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol 460: 563–572.
Eichenbaum H, Otto T, Cohen NJ (1992). The hippocampus—what does it do? Behav Neural Biol 57: 2–36.
Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ (2000). Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA 97: 7579–7584.
Feltenstein MW, See RE (2008). The neurocircuitry of addiction: an overview. Br J Pharmacol 154: 261–274.
Garcia-Fuster MJ, Flagel SB, Mahmood ST, Mayo LM, Thompson RC, Watson SJ et al (2011). Decreased proliferation of adult hippocampal stem cells during cocaine withdrawal: possible role of the cell fate regulator FADD. Neuropsychopharmacology 36: 2303–2317.
George O, Mandyam CD, Wee S, Koob GF (2008). Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology 33: 2474–2482.
Gerlai R (1998). A new continuous alternation task in T-maze detects hippocampal dysfunction in mice. A strain comparison and lesion study. Behav Brain Res 95: 91–101.
Gerlai R (2001). Behavioral tests of hippocampal function: simple paradigms complex problems. Behav Brain Res 125: 269–277.
Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999). Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2: 260–265.
Graybiel AM, Moratalla R, Robertson HA (1990). Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA 87: 6912–6916.
Gross NB, Marshall JF (2009). Striatal dopamine and glutamate receptors modulate methamphetamine-induced cortical Fos expression. Neuroscience 161: 1114–1125.
Hart CL, Ward AS, Haney M, Nasser J, Foltin RW (2003). Methamphetamine attenuates disruptions in performance and mood during simulated night-shift work. Psychopharmacology (Berl) 169: 42–51.
Hildebrandt K, Teuchert-Noodt G, Dawirs RR (1999). A single neonatal dose of methamphetamine suppresses dentate granule cell proliferation in adult gerbils which is restored to control values by acute doses of haloperidol. J Neural Transm 106: 549–558.
Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T (2006). Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci USA 103: 8523–8527.
Hiranita T, Nawata Y, Sakimura K, Yamamoto T (2008). Methamphetamine-seeking behavior is due to inhibition of nicotinic cholinergic transmission by activation of cannabinoid CB1 receptors. Neuropharmacology 55: 1300–1306.
Holscher C, Schmidt WJ (1994). Quinolinic acid lesion of the rat entorhinal cortex pars medialis produces selective amnesia in allocentric working memory (WM), but not in egocentric WM. Behav Brain Res 63: 187–194.
Hope B, Kosofsky B, Hyman SE, Nestler EJ (1992). Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci USA 89: 5764–5768.
Jessberger S, Clark RE, Broadbent NJ, Clemenson Jr GD, Consiglio A, Lie DC et al (2009). Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem 16: 147–154.
Johnson BA, Ait-Daoud N, Wells LT (2000). Effects of isradipine, a dihydropyridine-class calcium channel antagonist, on D-methamphetamine-induced cognitive and physiological changes in humans. Neuropsychopharmacology 22: 504–512.
Johnson BA, Roache JD, Ait-Daoud N, Wallace C, Wells LT, Wang Y (2005). Effects of isradipine on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition. Psychopharmacology (Berl) 178: 296–302.
Jones HE, Garrett BE, Griffiths RR (2001). Reinforcing effects of oral cocaine: contextual determinants. Psychopharmacology (Berl) 154: 143–152.
Kalivas PW, McFarland K (2003). Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168: 44–56.
Kee N, Sivalingam S, Boonstra R, Wojtowicz JM (2002). The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 115: 97–105.
Kim WR, Christian K, Ming GL, Song H (2011). Time-dependent involvement of adult-born dentate granule cells in behavior. Behav Brain Res; e-pub ahead of print.
Kim YT, Lee JJ, Song HJ, Kim JH, Kwon DH, Kim MN et al (2010). Alterations in cortical activity of male methamphetamine abusers performing an empathy task: fMRI study. Hum Psychopharmacol 25: 63–70.
King VR, Corwin JV (1992). Spatial deficits and hemispheric asymmetries in the rat following unilateral and bilateral lesions of posterior parietal or medial agranular cortex. Behav Brain Res 50: 53–68.
Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A et al (2004). Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev 27: 739–749.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Lalonde R (2002). The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26: 91–104.
Lasseter HC, Xie X, Ramirez DR, Fuchs RA (2010). Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience 171: 830–839.
Leuner B, Gould E, Shors TJ (2006). Is there a link between adult neurogenesis and learning? Hippocampus 16: 216–224.
Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G (2011). Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science 333: 353–357.
Mandyam CD, Harburg GC, Eisch AJ (2007a). Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience 146: 108–122.
Mandyam CD, Norris RD, Eisch AJ (2004). Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J Neurosci Res 76: 783–794.
Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF (2008). Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry 64: 958–965.
Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF (2007b). Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci 27: 11442–11450.
Moratalla R, Elibol B, Vallejo M, Graybiel AM (1996). Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron 17: 147–156.
Morgan D, Roberts DC (2004). Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev 27: 803–812.
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683.
Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF (2000). Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20: 798–805.
Nestler EJ (2001). Molecular neurobiology of addiction. Am J Addict 10: 201–217.
Nieto-Escamez FA, Sanchez-Santed F, de Bruin JP (2002). Cholinergic receptor blockade in prefrontal cortex and lesions of the nucleus basalis: implications for allocentric and egocentric spatial memory in rats. Behav Brain Res 134: 93–112.
Noonan MA, Bulin SE, Fuller DC, Eisch AJ (2010). Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci 30: 304–315.
Noonan MA, Choi KH, Self DW, Eisch AJ (2008). Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci 28: 2516–2526.
Parrott AC, Gibbs A, Scholey AB, King R, Owens K, Swann P et al (2011). MDMA and methamphetamine: some paradoxical negative and positive mood changes in an acute dose laboratory study. Psychopharmacology (Berl) 215: 527–536.
Paxinos G, Watson C (1997). The Rat Brain in Stereotaxic Coordinates, 3rd edn Academic Press: San Diego.
Rademacher DJ, Kovacs B, Shen F, Napier TC, Meredith GE (2006). The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur J Neurosci 24: 2089–2097.
Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE (2011). Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36: 782–792.
Ricoy UM, Martinez Jr JL (2009). Local hippocampal methamphetamine-induced reinforcement. Front Behav Neurosci 3: 47.
Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H et al (1999). Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci 2: 898–905.
Rogers JL, De Santis S, See RE (2008). Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 199: 615–624.
Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K et al (1999). Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 20: 322–339.
Ruskin DN, Marshall JF (1994). Amphetamine- and cocaine-induced fos in the rat striatum depends on D2 dopamine receptor activation. Synapse 18: 233–240.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809.
Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH et al (2010). Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage 50: 1392–1401.
Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH et al (2007). Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17: 275–297.
Scoville WB, Milner B (2000). Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci 12: 103–113.
Shen F, Meredith GE, Napier TC (2006). Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus. J Neurosci 26: 11041–11051.
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372–376.
Silber BY, Croft RJ, Papafotiou K, Stough C (2006). The acute effects of d-amphetamine and methamphetamine on attention and psychomotor performance. Psychopharmacology (Berl) 187: 154–169.
Silverman K, Kirby KC, Griffiths RR (1994). Modulation of drug reinforcement by behavioral requirements following drug ingestion. Psychopharmacology (Berl) 114: 243–247.
Simon SL, Dean AC, Cordova X, Monterosso JR, London ED (2010). Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs 71: 335–344.
Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR (2005). Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berl) 182: 186–193.
Sudai E, Croitoru O, Shaldubina A, Abraham L, Gispan I, Flaumenhaft Y et al (2011). High cocaine dosage decreases neurogenesis in the hippocampus and impairs working memory. Addict Biol 16: 251–260.
Tani K, Iyo M, Matsumoto H, Kawai M, Suzuki K, Iwata Y et al (2001). The effects of dentate granule cell destruction on behavioural activity and Fos protein expression induced by systemic methamphetamine in rats. Br J Pharmacol 134: 1411–1418.
Teuchert-Noodt G, Dawirs RR, Hildebrandt K (2000). Adult treatment with methamphetamine transiently decreases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm 107: 133–143.
Ursin R, Ursin H, Olds J (1966). Self-stimulation of hippocampus in rats. J Comp Physiol Psychol 61: 353–359.
van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96: 13427–13431.
Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL (2001). Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292: 1175–1178.
Wall PM, Messier C (2001). The hippocampal formation—orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behav Brain Res 127: 99–117.
Yan Y, Yamada K, Nitta A, Nabeshima T (2007). Transient drug-primed but persistent cue-induced reinstatement of extinguished methamphetamine-seeking behavior in mice. Behav Brain Res 177: 261–268.
Yuan CJ, Quiocho JM, Kim A, Wee S, Mandyam CD (2011). Extended access methamphetamine decreases immature neurons in the hippocampus which results from loss and altered development of neural progenitors without altered dynamics of the S-phase of the cell cycle. Pharmacol Biochem Behav 100: 98–108.
Zhou LF, Zhu YP (2006). Changes of CREB in rat hippocampus, prefrontal cortex and nucleus accumbens during three phases of morphine induced conditioned place preference in rats. J Zhejiang Univ Sci B 7: 107–113.
Acknowledgements
The study was supported by funds from the National Institute on Drug Abuse (DA022473 to CDM, DA010072 to GFK, and summer research fellowship to AHS), Pearson Center for Alcoholism and Addiction Research to GFK, NIH/Eunice Kennedy Shriver National Institute of Child Health & Human Development (T35HD064385 to PR), and Skaggs School of Pharmacy and Pharmaceutical Sciences (Skaggs Scholarship to CJY). We acknowledge the excellent technical assistance of Roxanne Kotzebue and Cameron Muhlfeld from the independent study program at the University of California, San Diego, and the San Diego State University, respectively, and Siddharth Iyengar and Wednesday Bushong from the Life Sciences Summer Internship Program at The Scripps Research Institute for assistance with immunohistochemistry and the cognitive behavior study. We appreciate the technical support of Elena Crawford and Robert Lintz and the editorial assistance of Michael Arends. The authors report no biomedical financial interests or potential conflict of interest. The publication number 21357 is from The Scripps Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that, except for the income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Recinto, P., Samant, A., Chavez, G. et al. Levels of Neural Progenitors in the Hippocampus Predict Memory Impairment and Relapse to Drug Seeking as a Function of Excessive Methamphetamine Self-Administration. Neuropsychopharmacol 37, 1275–1287 (2012). https://doi.org/10.1038/npp.2011.315
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.315