Abstract
The wars in Iraq and Afghanistan are associated with high rates of post-traumatic stress disorder (PTSD) and comorbid alcohol use disorders. The pharmacotherapy of these comorbid conditions has received relatively little study. The current study compared the serotonin uptake inhibitor, paroxetine, to the norepinephrine uptake inhibitor, desipramine. It also evaluated the adjunctive efficacy of the Food and Drug Administration (FDA)-approved alcoholism pharmacotherapy, naltrexone, relative to placebo. Four groups of predominately male veterans (n=88) meeting current diagnostic criteria for both alcohol dependence (AD) and PTSD were randomly assigned under double-blind conditions to one of four groups: paroxetine+naltrexone; paroxetine+placebo; desipramine+naltrexone; desipramine+placebo. Main outcome measures included standardized scales that assessed symptoms of PTSD and alcohol consumption. Paroxetine did not show statistical superiority to desipramine for the treatment of PTSD symptoms. However, desipramine was superior to paroxetine with respect to study retention and alcohol use outcomes. Naltrexone reduced alcohol craving relative to placebo, but it conferred no advantage on drinking use outcomes. Although the serotonin uptake inhibitors are the only FDA-approved medications for the treatment of PTSD, the current study suggests that norepinephrine uptake inhibitors may present clinical advantages when treating male veterans with PTSD and AD. However, naltrexone did not show evidence of efficacy in this population. This study was registered with ClinicalTrials.gov, registration number NCT00338962 and URL: http://clinicaltrials.gov/ct2/show/NCT00338962?term=desipramine+AND+alcohol+dependence+AND+depression&recr=Closed&rank=1.
Similar content being viewed by others
INTRODUCTION
Exposure to combat is a risk factor for mental health problems, notably the development of post-traumatic stress disorder (PTSD). The Iraq (Operation Iraqi Freedom) and Afghanistan (Operation Enduring Freedom) wars are the largest ground operations since the Vietnam War, and veterans from these recent conflicts have prevalence rates of PTSD between 6–12% (Erbes et al, 2007; Hoge et al, 2004, 2006). Among those seeking mental health care at the Veterans Administration nationally, PTSD accounted for 52% of the mental health diagnoses overall, and 13% of the diagnoses in Operation Enduring Freedom/Operation Iraqi Freedom veterans (Seal et al, 2007). Psychiatric comorbidity is an important factor in the treatment of PTSD. It is estimated that 80% of individuals with PTSD also suffer from another Axis I psychiatric disorder (Foa et al, 2000). In men, the most common co-occurring disorder is alcohol abuse or dependence (51.9%), and in women, it is the fourth most common diagnosis (27.9%; Fear et al, 2010; Kessler et al, 1995). Those individuals with comorbid alcohol dependence (AD) report more symptoms of PTSD in all three clusters (re-experiencing, avoidance, and hyperarousal) compared with those individuals with PTSD alone (Read et al, 2004; Riggs et al, 2003; Saladin et al, 1995). The comorbidity of PTSD and alcoholism is also associated with poorer alcohol-related outcomes, greater impairment in vocational functioning and quality of life, and increased risk for suicide (Brief et al, 2004; Johnson, 2008; Keane et al, 1988; Kosten and Krystal, 1988). Although there are approved medications for both PTSD and AD alone, the efficacy of these medications in comorbid populations is unclear. Given the high need for effective treatments for patients with comorbidity, evaluating the efficacy of pharmacotherapies for this patient population is a high clinical priority.
Serotonergic reuptake inhibitors (SRI) are the first-line treatment for PTSD, but there is a paucity of data comparing their efficacy to alternative treatment mechanisms. The SRIs paroxetine and sertraline are the only Food and Drug Administration (FDA)-approved pharmacotherapies for PTSD. The efficacy of these medications may be more robust in non-veteran and predominately female patient populations (Hertzberg et al, 1999; Smajkic et al, 2001), including a negative clinical trial in predominately male veterans (Friedman et al, 2007). Although an open-label study suggested that sertraline reduced PTSD symptoms and alcohol consumption (Brady et al, 1995), a larger randomized, placebo controlled study (n=94) showed no significant overall effect of sertraline on symptoms of PTSD or alcohol consumption (Brady and Sinha, 2005).
Noradrenergic mechanisms are implicated in the pathophysiology of PTSD (Krystal and Neumeister, 2009), but norepinephrine reuptake inhibitors (NRI) have not been tested adequately for military-related PTSD. A small, negative trial with desipramine in combat-related PTSD (Reist et al, 1989) was followed by a randomized comparison that showed the NRI reboxetine was of equal efficacy to an SRI (Spivak et al, 2006).
To date, there are no definitive trials of the combinatorial efficacy of antidepressants and the FDA-approved pharmacotherapies for alcoholism (acamprosate, naltrexone, and disulfiram). One study in veterans (n=254) found that naltrexone, disulfiram, or the combination of naltrexone and disulfiram did not differ from each other in efficacy, but were superior to placebo in improving drinking outcomes among alcohol-dependent patients with PTSD (Petrakis et al, 2006). Patients in this study remained on stable psychiatric pharmacotherapy regimens typical for PTSD, but this study did not randomly assign the PTSD medications, limiting the ability to explore the interactive effects of pharmacotherapies. Despite a negative clinical trial in veterans (Krystal et al, 2001), naltrexone is the only FDA-approved medication whose efficacy for alcoholism is supported by a large multi-center clinical trial conducted in the US (Anton et al, 2006).
The purpose of this study was to (1) evaluate and compare a noradrenergic antidepressant (desipramine) to the standard treatment for PTSD, with one of the FDA-approved serotonergic antidepressants (paroxetine) in a primarily veteran sample of participants with PTSD; (2) evaluate and compare their effectiveness in those participants with PTSD and comorbid AD, and (3) evaluate the value of the addition of the FDA-approved medication naltrexone in decreasing alcohol consumption.
SUBJECTS AND METHODS
Subjects
This study was approved by the Human Subjects Subcommittees of the VA Connecticut Healthcare System (West Haven, CT), and the Bedford VA Medical Center (Bedford, MA). Both VAs are affiliated with the New England Mental Illness Research Education and Clinical Center (MIRECC). The study was also approved by the Yale Human Investigations Committee (New Haven, CT). The present sample (n=88) consisted of veterans (n=81) and non-veterans (n=7). The participants were outpatients from the MIRECC-affiliated clinics (n=42 from CT, and n=46 from MA), who met DSM-IV criteria for current PTSD and AD, determined by structured clinical interview (First et al, 1996), who were abstinent no more than 29 days. Non-veterans in this sample were recruited through advertisements in the community and local newspapers. Exclusion criteria included unstable psychotic symptoms or serious current psychiatric symptoms, such as suicidal or homicidal ideation, or medical problems that would contraindicate the use of naltrexone, desipramine, or paroxetine, including liver function tests over three times the normal level. Subjects could not be taking medications thought to influence alcohol consumption (such as naltrexone, disulfiram, or acamprosate). Subjects were also required to be abstinent for 2 days before randomization (see Figure 1).
Flowchart describing the screening and randomization distribution for the participants in the study.
Treatments
After providing written informed consent, subjects completed an intake assessment, which included a physical examination, laboratory assessments, diagnostic, and psychiatric evaluation. Following completion of baseline assessments, 88 subjects were randomized to one of four groups for a 12-week trial. Randomization included (1) desipramine 200 mg per day or paroxetine 40 mg per day, and (2) randomization to naltrexone 50 mg or placebo in a double-blind fashion. This resulted in the following four groups, (1) paroxetine+naltrexone, (2) paroxetine+placebo, (3) desipramine+naltrexone, and (4) desipramine+placebo. Study medications were dispensed in blister packs (during the 2-week titration period for antidepressants) and packaged in separate bottles (after titration for 10 weeks), so subjects received two bottles, one labeled ‘naltrexone/antidepressant study medication’ and the other ‘naltrexone study medication’. Desipramine was started at a dose of 25 mg per day. The dose was gradually increased over 2 weeks to 200 mg per day. Paroxetine was started at 10 mg per day and the dose was gradually increased over 2 weeks to 40 mg per day. Naltrexone was started at 25 mg the first day and 50 mg per day for the rest of the treatment. Medication compliance was monitored at every visit for each bottle. All subjects also received Clinical Management/Compliance Enhancement therapy (Carroll et al, 1998) administered by trained research personnel.
Assessments
Primary outcomes were measures of PTSD severity and alcohol use. PTSD symptom severity was assessed biweekly by the clinician administered PTSD scale for DSM-IV (CAPS; Blake et al, 1990). The Alcohol Dependence Scale (ADS; Skinner and Horn, 1984) was administered at baseline to characterize the severity of AD. The Substance Abuse Calendar, based on the Timeline Followback Interview (Sobell and Sobell, 1992), was administered by highly trained research personnel at each weekly visit to collect a detailed self-report of daily alcohol and other substance use throughout the 84-day treatment period, as well as for the 90-day period before randomization. Alcohol consumption was confirmed using serum gamma-glutamyl transferase (GGT), collected four times during the study (baseline, week 4, week 8, and week 12). Craving was assessed weekly using the Obsessive Compulsive Drinking and Abstinence Scale (OCDS; Anton et al, 1996).
Side effects and common adverse symptoms were evaluated by the research staff weekly, using a modified version of the Systematic Assessment for Treatment Emergent Events (Levine and Schooler, 1986). The symptoms that are known to be associated with treatment with desipramine, paroxetine, and naltrexone were specifically screened for on a weekly basis. The symptoms were then clustered into the following categories: gastrointestinal, emotional, cold and flu symptoms, skin, sexual, neurological, and cardiac.
Data Analysis
Descriptive statistics were used to summarize the data on all randomized subjects. All continuous variables were examined for adherence to the normal distribution using normal probability plots and Kolmogorov–Smirnov tests. The alcohol data was not normally distributed. Log transformations were applied, but normality was not achieved. Therefore, the alcohol data was ranked and nonparametric tests for repeated measures analysis were used (Brunner et al, 2002). Baseline demographic characteristics for the four treatment groups (paroxetine+naltrexone, paroxetine+placebo, desipramine+naltrexone, desipramine+placebo) were compared using χ2-tests for categorical variables, and using analysis of variance for continuous variables. The analyses were on the intent-to-treat sample. The nonparametric, repeated measures analysis for the drinking data was performed in SAS Version 9.1.3. All other analyses were performed using 17.0 Version of SPSS. All statistical testing was at a two-tailed alpha level of 0.05. Bonferonni adjustments were applied to the analysis of CAPS subscales (three subscales; α=0.016), the alcohol data (three drinking outcome measures; α=0.016), craving data (two subscales: α=0.025), and the comparison of side effects (seven symptom groups; α=0.007).
The outcome variables included: a) PTSD symptoms (change from baseline CAPS total scores and change from baseline scores on CAPS subscales); and b) measures of alcohol consumption (average number of drinks per week, percent heavy drinking days, and drinks per drinking days) and craving (OCDS scores). Mixed-effects models were used to assess changes in PTSD symptoms and alcohol consumption over time. To understand better the differences among the four treatment groups, data were analyzed comparing desipramine to paroxetine, naltrexone to placebo, and their interaction in the same model. The treatment comparisons were between-subject factors in the models, and time (12 weeks) was used as a within-subject factor (when applicable). The use of the mixed-effects models approach for the analysis of our data has several specific advantages. Unlike traditional repeated measures analyses, mixed-effects models allow for different numbers of observations per subject, use of all available data on each subject, and are unaffected by randomly missing data. They also provide flexibility in modeling the correlation structure of the data (Gueorguieva and Krystal, 2004).
RESULTS
Demographic Characteristics
The subjects for this study were participants (n=88) who met current DSM-IV criteria for PTSD and AD (see Table 1). The participants were middle aged (mean age=47.1, SD=8.9), predominately Caucasian (75%), and male (90.1%), and the majority were either separated or divorced (59.1%; please see Table 1). There were no differences in age, sex, ethnicity, or marital status, based on treatment assignment in this sample. However, there were site differences on the basis of ethnicity (χ12=8.3, p=0.015), with 62% (n=26/42) Caucasians of the sample in West Haven in comparison with 87% (n=40/46) of the sample in Bedford. The sample consisted primarily of veterans (81/88 or 92%) and some non-veterans (7/88 or 8%). Participants were not on any psychiatric medications before starting study, with the exception of sleep medications that were taken as needed (n=6).
For the drinking baseline variables, data was collected for 90 days. Comparisons were made for each drinking variable (number of drinking days, drinks per drinking days, heavy drinking days, and % heavy drinking days) over each 30-day period (three times). As there were no differences in the overall drinking patterns over the three separate months before randomization, data is presented for the 30-day period before enrollment in the study. The participants in this study were heavy drinkers who drank on average 23.5 (SD=19.3) standard drinks on a drinking day, drank on average 19.9 (SD=9.9) days in a month, with more than half of their drinking days (mean=18.16, SD=10.7) in the past 30 days being heavy drinking days (five or more standard drinks). As shown in Table 1, there were no significant differences between the treatment groups at baseline in drinking days, drinks per drinking days, heavy drinking days, percent heavy drinking days, GGT levels, or ADS scores. However, there were site differences based on ADS scores (F1,84=4.44, p=0.038); participants in West Haven had lower ADS scores (mean=19.52, SD=1.32) than participants in Bedford (mean=23.41, SD=1.29).
Medication Dosing and Treatment Retention
Antidepressant medication was increased using a taper for all participants according to the predetermined schedule outlined earlier to a maximum dose of 200 mg for desipramine and 40 mg for paroxetine. Participants who could not tolerate the highest dose in either condition were brought to lower doses. In the group taking desipramine, 74% were tapered to the maximum dose of 200 mg, whereas in the group taking paroxetine, 79% were tapered to the maximum dose of 40 mg, indicating that the majority of the participants were able to tolerate the medications. The average maintenance dose for subjects that were on medication for at least 4 weeks was 187.2 (SD=31.7) for desipramine and 39.7 (SD=1.4) for paroxetine.
In this sample of individuals, 49/88 or 55.7% completed the entire trial. There were significantly more completers in the desipramine group (n=30/46, 65%) than in the paroxetine group (n=19/42, 45%; χ12=3.551, p=.05). Subjects in the desipramine group also took medication longer (mean=61.41 days, SD=31.2) than those in the paroxetine group (mean=45.48 days, SD=34.6; F1,84=4.99, p=0.02), and desipramine significantly increased the duration (days in the study) of study retention (χ12=3.7, p=0.053; please see Figure 2).
Survival curve of treatment retention comparing desipramine with paroxetine.
Naltrexone did not significantly influence the rate of study completion (χ12=.414, p=.52) and did not significantly increase the duration of study retention (naltrexone: 49.89±35.3 SD; placebo: 57.73 ±31.92; χ12=0.01, p=0.916). There were no significant differences among the treatment groups, based on rates of study retention (χ12=0.84, p=0.36).
PTSD Symptom Outcomes
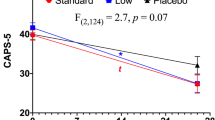
The CAPS data were analyzed using change from baseline CAPS scores in the model to control for baseline symptoms of PTSD (please see Table 2). There was a significant decrease in CAPS scores over time (F6108.8=2.175, p=0.051) and no significant interactions of treatment with time (desipramine/paroxetine by time F6108.8=1.249, p=0.287; naltrexone/placebo by time F6108.8=0.813, p=0.562), and no significant three-way interaction (please see Figure 3). Similarly, there was a significant decrease in each of the three CAPS subscale scores over time, but after Bonferonni adjustment was applied to the analysis (α=0.016), no treatment effects or interactions were significant.
Post-traumatic stress disorder (PTSD) symptoms measured using total clinician administered PTSD scale for DSM-IV (CAPS) scores over the entire 12 weeks of treatment for four treatment conditions.
Alcohol Use Outcomes
Relative to paroxetine, desipramine significantly reduced the percentage of heavy drinking days (F1.84=7.22, p=0.009) and drinks per drinking days (F1.84=5.04, p=0.027). Participants significantly reduced their weekly alcohol consumption during the study (time: ATS6.82=4.3, p=0.0001), and 51% of participants remained abstinent throughout the study. Also, after adjusting for multiple comparisons, there was a significant interaction for time by desipramine/paroxetine treatment on the number of drinks per week (ATS6.82=2.46, p=0.018; please see Figure 4), indicating that the desipramine-treated subjects had a greater reduction in their drinking over time compared with the paroxetine-treated subjects.
Number of standard drinks per week during pre-treatment (4 weeks) and during active treatment (12 weeks) for the four treatment conditions.
There were no significant effects of naltrexone on any of the drinking outcomes, including average number of drinks per week, percentage of heavy drinking days, and drinks per drinking days.
Craving
OCDS total scores decreased significantly over time (F12 104.8=6.87, p=0.0001). Naltrexone, relative to placebo, significantly decreased craving (F1582.0=6.39, p=0.012; naltrexone=19.88 (SD=12.89) at baseline, placebo=21.1 (SD=12.89) at baseline, compared with naltrexone=6.7 (SD=14.07) at endpoint, placebo=8.3 (SD=13.38) at endpoint). There were no significant differences in craving between the desipramine vs paroxetine-treated subjects (desipramine=20.0 (SD=12.61) at baseline, paroxetine=21.0 (SD=13.19) at baseline, compared with desipramine=6.1 (SD=13.09) at endpoint, and paroxetine=9.4 (SD=14.45) at endpoint) and no significant two-way or three-way interactions. OCDS (obsessions and compulsions) subscale scores decreased significantly over time. After Bonferonni adjustment was applied to the analysis (α=0.025), no treatment effects or interactions were significant.
GGT levels
GGT levels declined over time in the entire sample (F3121.8=3.3, p=0.022). This reduction was significantly greater in the desipramine-treated participants (F1229.5=5.08, p=0.02; desipramine baseline=55.2, paroxetine baseline=86.4; desipramine week 4=48.7, paroxetine week 4=46.1; desipramine week 8=41.7, paroxetine week 8=47.1; desipramine week 12=37.5, paroxetine week 12=57.1). Naltrexone did not significantly affect GGT levels.
Depression Symptom Outcomes
There was a significant decrease in the 17-item Hamilton Depression scores over time (F7405.6=8.57, p=0.0001) and no significant interactions of treatment with time (desipramine/paroxetine by time F7418.2=0.834, p=0.970; naltrexone/placebo by time F7418.1=0.152, p=0.994), and no significant three-way interaction (please see Table 2).
Adverse Events
There were five medical adverse events in this study; one in the desipramine+naltrexone group, two in the desipramine+placebo group, one in paroxetine+naltrexone group, and one in the paroxetine+placebo group. One study participant died in a work-related fatality that occurred 4 weeks after study medications were discontinued, and was deemed to be unrelated to the study. Among participants receiving desipramine, two reported dizziness or lightheadedness, one participant treated with this medication-developed tachycardia. These cases were managed by careful clinical monitoring and, in some cases, adjusting their desipramine dose. One participant in the paroxetine+placebo group experienced a seizure and study medication was discontinued.
There were four psychiatric adverse events in this study. Two participants were incarcerated related to alcohol intoxication (desipramine+placebo; paroxetine+naltrexone). One participant was hospitalized for severe anxiety (paroxetine+placebo). One participant (paroxetine+placebo) reported suicidal ideation 9 weeks after study medication was discontinued, which was deemed to be unrelated to study medication.
Side Effects
Desipramine-treated participants reported significantly more gastrointestinal symptoms (abdominal pain, nausea, vomiting, loss of appetite, constipation, diarrhea, aftertaste, dry mouth, coughing up blood, vomiting blood, black/bloody/light stool, yellow eyes, weight gain, and increased thirst) than paroxetine-treated subjects (F1.84=7.67, p=0.007). After adjustment for multiple comparisons (α=0.007), there were no other statistically significant differences in side effect reporting across the groups.
DISCUSSION
The current data provide the strongest evidence to date that NRIs might have a role in the treatment of individuals with PTSD and comorbid AD. In this study, desipramine had a comparable efficacy to paroxetine for PTSD symptoms, despite a prior literature questioning the efficacy of desipramine (Reist et al, 1989). Further, desipramine was superior in reducing alcohol consumption, as it significantly reduced the percentage of heavy drinking days and the number of drinks per week compared with paroxetine, confirmed by a significant decrease in GGT levels. However, there was no main effect of naltrexone on PTSD symptoms, and the combination of desipramine and naltrexone did not confer any clinical advantages.
Revisiting the role of NRIs in PTSD treatment is timely. Tricyclic antidepressants with NRI activity were among the first pharmacotherapies for PTSD supported by double-blind, placebo-controlled clinical trials (Frank and Thase, 1999). The superior tolerability profile for SRIs over tricyclic antidepressants, the emergence of definitive pharmacotherapy trials supporting the efficacy of SRIs for PTSD (Brady et al, 2000; Marshall et al, 2001; Tucker et al, 2001), the absence of definitive NRI trials for PTSD, and the negative preliminary findings with desipramine (Reist et al, 1989), all contributed to waning interest in NRI treatment for PTSD. More recently, the emergence of drugs that block the α1 noradrenergic receptor, such as prazosin (Raskind et al, 2007) and risperidone (Berger et al, 2009; Rothbaum et al, 2008) as promising treatments for the re-experiencing and hyperarousal symptoms of PTSD, converges with evidence of (a) the capacity of NRIs to attenuate noradrenergic activation (Charney and Heninger, 1985) and (b) of preliminary support for the NRI, reboxetine for PTSD (Spivak et al, 2006). These findings indicate that after 25 years of research, noradrenergic hyperactivity remains an important target for the treatment of PTSD (Van der Kolk, 2004).
The superior efficacy of desipramine to paroxetine for reducing heavy drinking may be particularly important for veterans with PTSD. SRIs appear to have mixed effects on drinking among patients diagnosed with PTSD, increasing drinking in some subgroups, whereas reducing drinking in others (Brady and Sinha, 2005). Similarly the efficacy of SRIs for reducing drinking among patients with comorbid depression has been questioned (Pettinati, 2001, 2004). NRIs have not been studied previously in patients with AD and PTSD, but a prior study provided suggestive evidence that desipramine reduced drinking only in alcohol-dependent patients with comorbid depression (Mason et al, 1996). Interestingly, the improved alcohol use outcomes were not reflected in improved symptoms of PTSD, but perhaps a longer study would address this issue.
In contrast to a prior report from our group (Petrakis et al, 2006), naltrexone did not reduce drinking in participants in this study. Differences in the design or execution of the current study may have influenced the opposing findings. The current study differs from the prior one in that participants were specifically recruited for this clinical trial on the basis of comorbid PTSD and AD. In contrast, the prior report was a secondary subgroup analysis of a larger study that recruited participants with a variety of psychiatric disorders. Also, participants in the prior study were on long-standing pharmacotherapies for PTSD, whereas participants in the current study either started or changed antidepressant medications while also being randomized to naltrexone or placebo. The failure to replicate the prior finding may be related to the smaller sample size and reduced statistical power in the current study. We acknowledge that our study was powered to detect medium to large effect sizes, and we do not have sufficient power to detect smaller effect sizes. The aforementioned study is consistent with secondary analyses of a prior VA multicenter study of naltrexone. This study reported that the increased risk for relapse to alcohol use among patients requiring the introduction of antidepressants for psychiatric symptoms may be mitigated by naltrexone (Krystal and Neumeister, 2009). Although naltrexone worsens symptoms associated with noradrenergic hyperactivity in human laboratory models (Charney and Heninger, 1986; Rosen et al, 1999), it did not worsen PTSD symptoms and in fact was well-tolerated by participants in this study.
Several issues limit conclusions that may be drawn from the current findings. First, the current findings may not generalize to all patients, such as women and non-veterans. Women may show superior SRI responses relative to tricyclic antidepressants for the treatment of both major depression and PTSD. The absence of an SRI and NRI efficacy difference in the current study may reflect the predominately male study sample. The results of the current study are consistent with other reports that evaluated the efficacy of an SRI for PTSD in predominately male veterans (without comorbidity) and showed no group differences in PTSD outcomes (Friedman et al, 2007). It should be noted that the studies differ in methodology, as one study used a placebo comparison whereas the current study used an active comparison. Second, drinking outcomes in SRI clinical trials for AD may be influenced by factors such as the age of onset of pathological drinking and of PTSD (Brady et al, 2000; Kranzler et al, 1996); these factors were not collected in this study. Third, this study compared desipramine and paroxetine with the objective of distinguishing the effects of NRI and SRI treatment for PTSD. However, desipramine is not truly a selective NRI. Recent evidence of the preliminary efficacy of prazosin in reducing PTSD symptoms (Raskind et al, 2007) suggests that a medication that has the capacity to block α1 adrenergic receptors (Richelson, 2003) may be related to its efficacy in treating symptoms of PTSD. This important issue limits the capacity of the current findings to inform the question as to whether highly selective NRIs would be preferred for the treatment of this population over medications that block both the norepinephrine transporter and α1 adrenergic receptors. Also, this study could not address the combinatorial impact of blockade of serotonin and norepinephrine transporters, ie, SNRIs. These drugs have shown preliminary efficacy for PTSD (Davidson et al, 2006; Pae et al, 2007; Richelson, 2003). Their efficacy in AD is untested.
Overall, this study provided support for further investigation of desipramine treatment for participants with comorbid PTSD and AD. Although paroxetine, but not desipramine, is FDA-approved for PTSD treatment, the current study did not find a significant difference between desipramine and paroxetine with respect to their effectiveness in treating PTSD symptoms. Further, in this sample of predominately male veterans, desipramine significantly reduced heavy drinking compared with paroxetine, making it an important possible therapeutic agent in treating this population of patients.
References
Anton RF, Moak DH, Latham PK (1996). The obsessive compulsive drinking scale: a new method of assessing outcome in alcoholism treatment studies (published erratum appears in Arch Gen Psychiatry 1996 Jul;53(7):576). Arch Gen Psychiatry 53: 225–231.
Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM et al (2006). Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. [see comment]. JAMA 295: 2003–2017.
Berger W, Mendlowics MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR et al (2009). Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry 33: 169–180.
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G et al (1990). A clinician rating scale for assessing current and lifetime PTSD: the CAPS-1. Behav Ther 13: 187–188.
Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR et al (2000). Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA 283: 1837–1844.
Brady K, Sonne S, Roberts J (1995). Sertraline treatment of comorbid post traumatic stress disorder and alcohol dependence. J Clin Psychiatry 56: 502–505.
Brady KT, Sinha R (2005). Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. [see comment]. Am J Psychiatry 162: 1483–1493.
Brief DJ, Bollinger AR, Vielhauer MJ, Berger-Greenstein JA, Morgan EE, Brady SM et al (2004). Understanding the interface of HIV, trauma, post-traumatic stress disorder, and substance use and its implications for health outcomes. AIDS Care 16: S97–S120.
Brunner E, Domhof S, Langer F (2002). Nonparametric Analysis of Longitudinal Data in Factorial Experiments. John Wiley & Sons: New York, NY.
Carroll KM, Nich C, Ball SA, McCance E, Rounsaville BJ (1998). Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction 93: 713–727.
Charney DS, Heninger GR (1985). Noradrenergic function and the mechanism of action of antianxiety treatment. The effect of long-term imipramine treatment. Arch Gen Psychiatry 42: 473–481.
Charney DS, Heninger GR (1986). Alpha 2-adrenergic and opiate receptor blockade. Synergistic effects on anxiety in healthy subjects. Arch Gen Psychiatry 43: 1037–1041.
Davidson JR, Baldwin D, Stein DJ, Kuper E, Benattia I, Ahmed S et al (2006). Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry 63: 1158–1165.
Erbes C, Westermeyer J, Engdahl B, Johnsen E (2007). Post-traumatic stress disorder and service utilization in a sample of service members from Iraq and Afghanistan. Mil Med 172: 359–363.
Fear NT, Jones M, Murphy D, Hull L, Sundin J, Iversen AC et al (2010). What are the consequences of deployment to Iraq and Afghanistan on the mental health of the UK armed forces? A cohort study. Lancet 375: 1783–1797.
First MB, Spitzer RL, Gibbon M, Williams JBW (1996). Structured Clinical Interview for DSM-IV Axis I Disorders (Patient Edition) (SCID-P). Biometric Research, New York State Psychiatric Institute, New York, NY.
Foa E, Keane TM, Friedman MJ (2000). The Effective Treatments for PTSD. The Guilford Press: New York.
Frank E, Thase ME (1999). Natural history and preventative treatment of recurrent mood disorders. Ann Rev Med 50: 453–468.
Friedman MJ, Marmar CR, Baker DG, Sikes CR, Farfel GM (2007). Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry 68: 711–720.
Gueorguieva R, Krystal J (2004). Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry 61: 310–317.
Hertzberg MA, Butterfield MI, Feldman ME, Beckham JC, Sutherland SM, Connor KM et al (1999). A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Biol Psychiatry 45: 1226–1229.
Hoge CW, Auchterlonie JL, Milliken CS (2006). Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA 295: 1023–1032.
Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL (2004). Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 351: 13–22.
Johnson SD (2008). Substance use, post-traumatic stress disorder and violence. Curr Opin Psychiatry 21: 242–246.
Keane TM, Gerardi RJ, Lyons JA, Wolfe J (1988). The interrelationship of substance abuse and posttraumatic stress disorder: epidemiological and clinical considerations. Recent Dev Alcohol 6: 27–48.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060.
Kosten TR, Krystal J (1988). Biological mechanisms in posttraumatic stress disorder: Relevance for substance abuse. Recent Dev Alcohol 6: 49–68.
Kranzler H, Burleson J, Brown J, Babor T (1996). Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res 20: 1534–1541.
Krystal J, Neumeister A (2009). Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resiliance. Brain Res 1293: 13–23.
Krystal JH, Cramer J, Krol W, Kirk G, Rosenheck R (2001). Naltrexone in the treatment of alcohol dependence. N Engl J Med 345: 1734–1739.
Levine J, Schooler NR (1986). Strategies for analyzing side effect data from SAFTEE: a workshop held fall 1985 in Rockville, Maryland. Psychopharmacol Bulletin 22: 343–356.
Marshall RD, Beebe KL, Oldham M, Zaninelli R (2001). Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry 158: 1982–1988.
Mason BJ, Kocsis JH, Ritvo EC, Cutler RB (1996). A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression [see comments]. JAMA 275: 761–767.
Pae CU, Lim HK, Ajwani N, Lee C, Patkar AA (2007). Extended-release formulation of venlafaxine in the treatment of post-traumatic stress disorder. Expert Rev Neurother 7: 603–616.
Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Ralevski E et al (2006). Naltrexone and disulfiram in patients with alcohol dependence and post traumatic stress disorder. Biol Psychiatry 60: 777–783.
Pettinati HM (2001). The use of selective serotonin reuptake inhibitors in treating alcoholic subtypes. J Clin Psychiatry 62 Suppl: 26–31.
Pettinati HM (2004). Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry 56: 785–792.
Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D et al (2007). A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. [see comment]. Biol Psychiatry 61: 928–934.
Read JP, Brown PJ, Kahler CW (2004). Substance use and posttraumatic stress disorders: symptom interplay and effects on outcome. Addict Behav 29: 1665–1672.
Reist C, Kauffmann CD, Haier RJ, Sangdahl C, DeMet EM, Chicz-DeMet A et al (1989). A controlled trial of desipramine in 18 men with posttraumatic stress disorder. Am J Psychiatry 146: 513–516.
Richelson E (2003). Interactions of antidepressants with neurotransmitter transporters and receptors and their clinical relevance. J Clin Psychiatry 45: 1037–1041.
Riggs DS, Rukstalis M, Volpicelli JR, Kalmanson D, Foa EB (2003). Demographic and social adjustment characteristics of patients with comorbid posttraumatic stress disorder and alcohol dependence: potential pitfalls to PTSD treatment. Addict Behav 28: 1717–1730.
Rosen MI, Kosten TR, Kreek MJ (1999). The effects of naltrexone maintenance on the response to yohimbine in healthy volunteers. Biol Psychiatry 45: 1636–1645.
Rothbaum BO, Killeen TK, Davidson JR, Brady KT, Connor KM, Heekin MH (2008). Placebo-controlled trial of risperidone augmentation for selective serotonin reuptake inhibitor-resistant posttraumatic stress disorder. J Clin Psychiatry 69: 520–525.
Saladin ME, Brady KT, Dansky BS, Kilpatrick DG (1995). Understanding comorbidity between PTSD and substance use disorders: two preliminary investigations. Addict Behav 20: 643–655.
Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C (2007). Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med 167: 476–482.
Skinner HA, Horn JL (1984). Alcohol Dependence Scale (ADS): User's Guide. Addiction Research Foundation: Toronto.
Smajkic A, Weine S, Djuric-Bijedic Z, Boskailo E, Lewis J, Pavkovic I (2001). Sertraline, paroxetine, and venlafaxine in refugee posttraumatic sress disorder with depression symptoms. J Trauma Stress 14: 445–452.
Sobell LC, Sobell MB (1992). Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. Measuring Alcohol Consumption: Psychosoical and biological methods. Litten, RZ and Allen, J. Humana Press: Totowa, NJ. pp 41–72.
Spivak B, Strous RD, Shaked G, Shabash E, Kotler M, Weizman A (2006). Reboxetine vs fluvoxamine in the treatment of motor vehicle accident-related posttraumatic stress disorder: a double-blind, fixed-dosage, controlled trial. J Clin Psychopharmacol 26: 152–156.
Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD (2001). Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry 62: 860–868.
Van der Kolk B (2004). Psychobiology of posttraumatic stress disorder. Textbook of Biological Psychiatry. Panksepp, J. Wiley-Liss: New York. pp 319–344.
Acknowledgements
This study was conducted with the invaluable help of the VA VISN I MIRECC Study Group: Department of Psychiatry, Bedford VAMC: Marylee Losardo, MSPA, Barbara E Rofman, RN, MS; Department of Psychology, Bedford VAMC: Charles E Drebing, PhD; Department of Psychiatry, VA CT Healthcare, West Haven Campus: Kathryn Keegan, RN, Diana Limoncelli, BA, Colette McHugh-Strong, JD, Alison Oville, BA, Christine Sicignano, BA, J Serrita Jane, PhD, Erin O’Brien, PsyD. Support was provided by VISN I Mental Illness Research Education and Clinical Center (MIRECC; PI, Rounsaville), the VA Alcohol Center (PI, Krystal), and Clinical Neuroscience Division of the VA National Center for PTSD (PI, Krystal).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Ismene L Petrakis, Dr Elizabeth Ralevski, Dr Nitigna Desai, Dr Louis Trevisan, and Dr Ralitza Gueorguieva declare no conflict of interest. Dr John H Krystal (during the period 2008–2011) has served as a scientific consultant to the following companies (The Individual Consultant Agreements listed below are less than $10 000 per year): Aisling Capital, LLC AstraZeneca Pharmaceuticals, Biocortech, Brintnall & Nicolini, Easton Associates, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck Research USA, Medivation, Merz Pharmaceuticals, MK Medical Communications, F Hoffmann-La Roche, SK Holdings, Sunovion Pharmaceuticals, Takeda Industries, Teva Pharmaceutical Industries. He is on the Scientific Advisory Board for the following companies: Abbott Laboratories, Bristol-Myers Squibb, Eisai, Eli Lilly, Forest Laboratories, Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Naurex, Pfizer Pharmaceuticals, Shire Pharmaceuticals. He holds less than $150 in exercisable warrant options with Tetragenex Pharmaceuticals. He is on the Board of Directors: Coalition for Translational Research in Alcohol and Substance Use Disorders. He is President Elect: American College of Neuropsychopharmacology. He is the principal investigator of a multicenter study in which Janssen Research Foundation has provided drug and some support to the Department of Veterans Affairs. He is on the Editorial Board, Editor of Biological Psychiatry (Income Greater than $10 000). He has Patents and Inventions: 1) Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. Patent number: 5 447 948, 5 September 1995; I am a co-inventor with Dr Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1). Intranasal Administration of Ketamine to Treat Depression (pending).
Rights and permissions
About this article
Cite this article
Petrakis, I., Ralevski, E., Desai, N. et al. Noradrenergic vs Serotonergic Antidepressant with or without Naltrexone for Veterans with PTSD and Comorbid Alcohol Dependence. Neuropsychopharmacol 37, 996–1004 (2012). https://doi.org/10.1038/npp.2011.283
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.283
Keywords
This article is cited by
-
Selective serotonin reuptake inhibitors in the treatment of depression, anxiety, and post-traumatic stress disorder in substance use disorders: a Bayesian meta-analysis
European Journal of Clinical Pharmacology (2022)
-
Dealing With Complexity and Comorbidity: Comorbid PTSD and Substance Use Disorders
Current Treatment Options in Psychiatry (2019)
-
Augmenting Treatment for Posttraumatic Stress Disorder and Co-Occurring Conditions with Oxytocin
Current Treatment Options in Psychiatry (2019)
-
Old Friends, immunoregulation, and stress resilience
Pflügers Archiv - European Journal of Physiology (2019)
-
Therapeutic challenges for concurrent ethanol and nicotine consumption: naltrexone and varenicline fail to alter simultaneous ethanol and nicotine intake by female alcohol-preferring (P) rats
Psychopharmacology (2019)