Abstract
Modafinil differs from other arousal-enhancing agents in chemical structure, neurochemical profile, and behavioral effects. Most functional neuroimaging studies to date examined the effect of modafinil only on information processing underlying executive cognition, but cognitive enhancers in general have been shown to have pronounced effects on emotional behavior, too. We examined the effect of modafinil on neural circuits underlying affective processing and cognitive functions. Healthy volunteers were enrolled in this double-blinded placebo-controlled trial (100 mg/day for 7 days). They underwent BOLD fMRI while performing an emotion information-processing task that activates the amygdala and two prefrontally dependent cognitive tasks—a working memory (WM) task and a variable attentional control (VAC) task. A clinical assessment that included measurement of blood pressure, heart rate, the Hamilton anxiety scale, and the profile of mood state (POMS) questionnaire was also performed on each test day. BOLD fMRI revealed significantly decreased amygdala reactivity to fearful stimuli on modafinil compared with the placebo condition. During executive cognition tasks, a WM task and a VAC task, modafinil reduced BOLD signal in the prefrontal cortex and anterior cingulate. Although not statistically significant, there were trends for reduced anxiety, for decreased fatigue-inertia and increased vigor-activity, as well as decreased anger-hostility on modafinil. Modafinil in low doses has a unique physiologic profile compared with stimulant drugs: it enhances the efficiency of prefrontal cortical cognitive information processing, while dampening reactivity to threatening stimuli in the amygdala, a brain region implicated in anxiety.
Similar content being viewed by others
INTRODUCTION
Psychostimulants and other putative arousal-enhancing drugs have a wide range of potential treatment targets in neuropsychiatry including cognitive dysfunction, which is a core feature of a broad range of neuropsychiatric disorders. Of the different arousal-enhancing agents currently available, modafinil is gaining increasing popularity because of its relatively lower liability to abuse (Deroche-Gamonet et al, 2002) and a lower risk of adverse effects on the cardiovascular system (Makris et al, 2004; Jr et al, 2009). Converging evidence from studies in animal models and in humans suggests that the different behavioral effects induced by modafinil compared with traditional psychostimulants are due to differences in structure and neurochemical profile. Evidence indicates that modafinil directly binds and inhibits both the dopamine transporter (DAT) and norepinephrine transporter (NET) with modest potency leading to significant but relatively small elevations in extracellular dopamine (DA) and norepinephrine (NE) levels (Madras et al, 2006). Modafinil administration has also been shown to decrease γ-aminobutyric acid (GABA) levels and elevate levels of serotonin (5HT), glutamate, orexin, and histamine, possibly secondary to catecholamine effects (Minzenberg and Carter, 2008; Qu et al, 2008).
Although there has been considerable effort to define the effects of modafinil as a cognitive enhancer (Minzenberg and Carter, 2008; Wesensten, 2006), its effect at the level of limbic system function and on the information processing underlying emotion regulation has been relatively unexamined. This is an important issue of clinical relevance as other cognitive enhancers such as amphetamine have been shown to have a pronounced effect on emotional behavior, including the generation of fear and anxiety (Angrist and Gershon, 1970; Ellinwood et al, 1973; Hall et al, 1988) along with exaggerated amygdala reactivity during the perceptual processing of angry and fearful facial expressions (Hariri et al, 2002). Data from animal studies have shown either no effects of modafinil on anxiety scales (Hermant et al, 1991; Simon et al, 1994) or an anxiolytic effect (van Vliet et al, 2006). Results from studies conducted on humans are less consistent; some studies show either an anxiolytic effect (Becker et al, 2004), or no effect on anxiety ratings (Saletu et al, 2007; Samuels et al, 2006), while others report an anxiogenic effect (Broughton et al, 1997; MacDonald et al, 2002; Schwartz et al, 2003; Taneja et al, 2007; Zifko et al, 2002). However, all these studies vary in doses used (100 mg, 200 mg, or 400 mg) and in the dosing schedule (one time vs chronic dosing over a week or more), which may account for the discrepancy in results. This possibility is consistent with evidence that shows that repeated administration of modafinil in monkeys results in a decrease in motor hyperactivity observed after single-dose administration (Hermant et al, 1991).
In this study, we examined the effect of chronic low-dose modafinil (100 mg/day for 1 week) on both emotion information-processing as well as cognitive information-processing circuits in a sample of healthy non-sleep-deprived volunteers. Subjects were assessed with fMRI while they performed an emotion information-processing task that has previously been shown to reliably activate the limbic circuit including the amygdala (Hariri et al, 2002), and two prefrontally dependent cognitive tasks well known to engage the prefrontal cortex (PFC) (Callicott et al, 2003) and anterior cingulate (Blasi et al, 2005). On the basis of previous evidence for decreased anxiety (Provigil website http://www.provigil.com/media/PDFs/prescribing_info.pdf) with improved cognition on modafinil, we hypothesized that this would be reflected at the level of neurobiology either as no change in amygdala reactivity or as decreased amygdala reactivity on modafinil during the emotion-processing task while coincidentally, modafinil would improve cortical efficiency during cognitive information processing. Improved cortical efficiency can be defined as engaging less cortical activity or more focused cortical activity on modafinil for a similar level of task performance as during the placebo session. Controlling for task performance across diagnosis, genotype groups, drug conditions, and so on, is an approach that has been reliably used in a number of studies to circumvent the confound associated with performance related differences in neural activity (Tan et al, 2007). Improved efficiency during executive cognitive processing has been reported to be a characteristic effect of other psychostimulant drugs (eg, Mattay et al, 2003).
MATERIALS AND METHODS
A randomized, double-blind, placebo-controlled, crossover design was used to study 38 healthy control subjects who were recruited from local and national resources as volunteers for the ‘CBDB/NIMH Sibling Study’ (Table 1a). Written informed consent was obtained from the subjects after complete description of the study, to be part of the drug protocol (# 03-M-0143) approved by the National Institute of Mental Health Institutional Review Board for administration of oral modafinil. All participants underwent a structured clinical interview to rule out an active axis I or axis II diagnosis (DSM-IV) (APA, 1994) that could potentially bias the results. Exclusion criteria are reported in Supplementary Materials and Methods. Four subjects did not complete the second arm of the study. Several other subjects—varying according to the task—were excluded because of excessive movement or technical problems during either the drug or placebo sessions. The fMRI analysis, therefore, was limited to data from subjects with fMRI data from both days that passed a rigorous quality control check (all fMRI data were individually examined for motion artifacts and we excluded from further analysis data from subjects with excessive inter-scan motion: >2 mm translation, >1.5° rotation on either the placebo or drug session). This resulted in 19 subjects with usable BOLD fMRI data for the face-matching task (FMT), 23 subjects with usable data during the 2-back task, and 11 subjects with usable data for the variable attentional control (VAC) task on both days (the VAC task was added to the protocol half-way through the study) (Table 1b). Paired t-tests between placebo and modafinil sessions showed no significant difference in the inter-scan movement parameters (mean and max values all p>0.1) and the signal-to-noise ratio values for the fMRI time series images (all p>0.5) for each of the three tasks. There were no differences in behavioral measures as evaluated by Hamilton anxiety scale (HAM-A) (Hamilton, 1959) and the profile of mood state (POMS) questionnaire (McNair et al, 1992) between the subjects included in the three fMRI analyses and the subjects excluded (all p>0.07).
Drug Administration
A table of random numbers was used to prepare the randomization. Both modafinil and placebo were coded. Coded modafinil (100 mg once daily) was administered orally every morning for 7 days. After a 1-week wash out period following the first arm, subjects who had received coded modafinil during the first arm received coded placebo, while those who started on coded placebo during the first arm received 100 mg of coded modafinil. The order of drug for each task is reported in Table 1b. The capsules of modafinil and placebo were identical in appearance (pink color) and taste. Side effects were minimal or absent at the dose used and no subject discontinued the protocol because of side effects.
Functional Assessment
Functional assessment was performed on days 7 and 21. fMRI was started ≈180 min after drug administration (placebo or modafinil), and completed within 4 h after administration. Timing of testing was based on pharmacokinetic data indicating that plasma levels of modafinil peak 2–4 h after oral administration (Robertson and Hellriegel, 2003). Three hours after drug administration, just before the scan, a blood sample was obtained for serum modafinil levels (measured at Cephalon Inc., Frazer, PA, using high-performance liquid chromatography—Gorman, 2002). A clinical assessment including blood pressure and heart rate was performed before the scan. Each subject also completed the HAM-A scale and the POMS questionnaire to determine mood, anxiety, and energy on each test day.
Tasks and Data Acquisition
The FMT, the 2-back task, and the VAC task have been described previously (Blasi et al, 2005; Callicott et al, 2003; Hariri et al, 2002).
Face-matching task
The blocked fMRI paradigm consists of two experimental conditions, an emotional face-matching condition and a sensorimotor control task. The task consisted of five blocks of 30-s duration each. Blocks 1, 3, and 5 were sensorimotor blocks and blocks 2 and 4 were emotion blocks. Each block of either type consisted of six trials, each of 5-s duration. Each trial consisted of the presentation of three images, two in the lower panel and one in the upper panel. In the six trials of each sensorimotor block, the two lower images were of shapes, and the upper panel image was identical to one of the shapes in the lower panel. Subjects responded with button presses (left or right) to indicate which of the two lower panel images matched the upper panel image. In the six trials of each emotion block, the lower panel consisted of two faces, one angry and one fearful, derived from a standard set of pictures of facial affect (Ekman and Friesen, 1976). The upper panel consisted of one of the two faces shown in the lower panel. Subject responded with button presses (left or right) to indicate which lower panel face matched the face in the upper panel.
2-Back task
The 2-back task consisted of presentation of visual stimuli in which a series of numbers (1–4) were presented randomly every 2000 ms for 500 ms at set locations at the points of a diamond-shaped box. Subjects were asked to encode the currently observed number and simultaneously recall the number observed two times previously, and respond through a MRI compatible button box, which had four buttons arranged in the same configuration as the stimuli presented on the screen. The task was presented as four blocks of control condition (0-back) alternating with four blocks of the 2-back condition.
VAC task
Each stimulus was composed of arrows of three different sizes pointing either to the right or to the left. Subjects were instructed by a cue word (BIG, MEDIUM, or SMALL) showed above each stimulus to press the right or left button corresponding to the direction of the large, medium, or small arrows. There were three different levels of attentional control: (1) low level of attentional control (LOW): all three sizes of arrows were congruent in direction with each other, and the stimuli were cued with the word BIG. (2) Intermediate level (INT): the big arrow was incongruent in direction to the small and the medium arrows; the cue was either BIG or SMALL. (3) High level (HIGH): the medium-sized arrows were incongruent in direction to the big and the small arrows; the cue was either SMALL or MEDIUM. In addition, a simple bold arrow pointing to either the left or right was used as a sensorimotor control condition of no conflict.
Each stimulus was presented for 800 ms, and the order of the stimuli was randomly distributed across the session (Friston et al, 1999). The total number of stimuli was 241: 50 HIGH, 68 INT, 57 LOW, and 66 simple bold arrows. A fixation cross-hair was presented during the interstimulus interval, which ranged from 2000 to 6000 ms.
Analysis of Imaging Data
Images were processed as described (Blasi et al, 2005; Callicott et al, 2003; Hariri et al, 2002) in SPM2 (www.fil.ion.ucl.ac.uk/spm). A complete description of the image analysis is reported in Supplementary Materials and Methods.
All results are reported in MNI coordinates and at a threshold of p<0.05 corrected for multiple comparisons based on family-wise error (FWE) within region of interests (ROIs) appropriately chosen according to the task (bilateral amygdala ROI for FMT; bilateral PFC ROI—encompassing BAs 46 and 47- for 2-back task, and anterior cingulate—ACC—for VAC task, defined using the Wake Forest University PICKATLAS toolbox, version 2.0 http://www.fmri.wfubmc.edu) (Supplementary Figure).
Functional Connectivity
We analyzed amygdala/supragenual-subgenual cingulate coupling that is hypothesized to modulate amygdala reactivity through top–down control (Pezawas et al, 2005). A complete description of the method used to assess functional connectivity is reported in Supplementary Materials and Methods.
Statistical Analysis on Clinical, Behavioral, and Performance Data
Paired t-test (heart rate, FMT performances, 2-back task performance, HAM-A, and POMS) and analysis of variance for repeated measures (blood pressure, VAC task performance) were performed using STATISTICA software (Statsoft Corp., Tulska, OK). Owing to a computer glitch, reaction time (RT) could not be collected for one subject during one of the two sessions for the 2-back task. Similarly, HAM-A scale and POMS questionnaire could not be completed on one of the 2 days in five subjects, therefore analyses were limited on data from subjects that had data available on both days. Modafinil levels were not available for three subjects included in 2-back and VAC task analyses and for five subjects included in FMT analysis. Bonferroni correction was performed for multiple testing.
Correlation Between Serum Modafinil Levels and Behavioral and Neuroimaging Data
To examine the relationship between serum modafinil levels and changes in behavioral measures (HAM-A scale and POMS questionnaire) and brain activity, we performed linear correlation analysis; Pearson's r was used for neuroimaging data, and a Spearman's rho correlation method was used for behavioral data as they did not follow a normal distribution. For behavioral data, correlation was carried out between serum modafinil levels and the difference scores between placebo and modafinil conditions on the HAM-A scale and POMS subscales.
For neuroimaging data, correlation analysis was carried out between serum modafinil levels and the mean signal extracted from a 10-mm radius spherical ROI around the peak voxel obtained from the contrast looking for a main effect of drug for each of the task conditions (amygdala for FMT, PFC, and ACC for 2-back and VAC task; ROI created using MarsBar).
RESULTS
Neuroimaging Data
Face-matching task
Consistent with previous evidence (Hariri et al, 2002), perceptual processing of fearful and angry facial expressions was associated with bilateral amygdala activity during both placebo and modafinil sessions (data not shown). A paired t-test analysis showed a significant effect of drug in the right amygdala with lower activation on modafinil when compared with placebo (peak voxel coordinates (x, y, z)=(26, 4, −20), Z=2.91, p=0.04 FWE-corrected within bilateral amygdala ROI, Figure 1).
FMT. Main effect of drug: placebo>modafinil (N=19). Effect of modafinil on amygdala reactivity during an implicit threatening stimuli-processing task mean parameter estimates extracted from a 10-mm radius spherical ROI created around the peak voxel in amygdala obtained from the contrast placebo>modafinil (peak voxel coordinates (x, y, z)=(26, 4, −20), Z=2.91, p=0.04 FWE-corrected). Bars show the mean.
Functional connectivity analysis performed using the right amygdala as the seed ROI revealed that there was significantly increased negative coupling between amygdala and supragenual ACC ((x, y, z)=(4, 38, 30), Z=2.84, p=0.05 FWE-corrected within the ROI) and significantly decreased positive coupling between amygdala and subgenual ACC ((x, y, z)=(0, 41, −10), Z=2.66, p=0.05 FWE-corrected within the ROI) on modafinil relative to placebo.
The coupling between subgenual ACC and supragenual ACC, positive during both the placebo and modafinil sessions, did not show any significant difference across drug sessions. Accuracy and RT during the FMT were similar between placebo and modafinil sessions (all p>0.2), which suggests that the subjects were attending to the task similarly during both placebo and modafinil sessions.
2-Back task
The spatial distribution of the activation responses during the 2-back task included the PFC, pericingulate cortex, anterior cingulate, and parietal cortex bilaterally on both drug sessions, as previously described (Callicott et al, 2003) (data not shown). A paired t-test revealed a significant main effect of drug with greater activation in the right PFC during placebo session compared with the modafinil session (peak voxel coordinates (x, y, z)=(38, 34, −6), Z=3.61, P=0.05 FWE-corrected within bilateral PFC ROI) (Figure 2). No significant PFC activation differences were observed on the reverse contrast (modafinil > placebo). Importantly, these changes in BOLD fMRI signal were observed in the absence of any difference in accuracy and RT during the 2-back working memory (WM) task (all p>0.2), and therefore reflect an improvement in prefrontal cortical efficiency on modafinil.
2-Back task. Main effect of drug: placebo>modafinil (N=23). Effect of modafinil on PFC activation during a WM task mean parameter estimates extracted from a 10-mm radius spherical ROI created around the peak voxel in PFC obtained from the contrast placebo>modafinil (peak voxel coordinates (x, y, z)=(38, 34, −6), Z=3.61, p=0.05 FWE-corrected). Bars show the mean.
VAC task
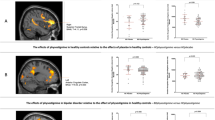
A significant main effect of task, that is, an effect of increase in demand for attentional control, was found in several regions, including the dorsolateral PFC, the anterior cingulate, the parietal cortex, the supplementary motor area, and the ventrolateral PFC bilaterally (data not shown), as previously described (Blasi et al, 2005). When looking at the effect of drug on the ‘level of attentional control’, we observed a significant drug-effect in anterior cingulate activation (Figure 3a). Specifically, when task load increases from low to intermediate to the high level of attention, subjects have higher activation in the anterior cingulate on placebo when compared with modafinil (peak voxel coordinates (x, y, z)=(11, 38, 25), Z=3.02, p=0.04 FWE-corrected within anterior cingulate ROI), in spite of the similar task performance across both sessions (Figure 3b).
VAC task: effect of modafinil on anterior cingulate activation during an attentional control task and behavioral data. Drug effect in the VAC task: (a) group statistical parametric maps illustrating a significant effect of modafinil in decreasing anterior cingulate activation when task load increases from low to intermediate to high level of attention when compared with placebo session. For illustrative purpose, map thresholded at p=0.01 uncorrected k>3; (b) behavioral data (mean ±95% confidence intervals). Accuracy: there was a significant main effect of increasing demand for attentional control (F(2, 20)=11, p<0.001), with subjects performing worse on the high demand condition relative to the intermediate and low demand conditions, both during placebo and modafinil session (b left). There was no significant main effect of drug or drug × condition interaction (all p>0.4). RT: there was a significant main effect of increasing demand for attentional control on RT during both placebo and modafinil sessions (F(2, 20)=96.9, p<0.001), with a statistically significant difference across all three levels of demand for attentional control (post hoc analyses using Fisher's least significant difference: all p<0.01). Although a decrease in RT was observed during modafinil at all three levels of demand (b right), the main effect of drug on RT as well as the drug × attentional load interaction were not statistically significant (all p>0.1).
Drug × gender interaction
No significant gender × drug interaction was found either in the behavioral data or neuroimaging data for all three tasks.
Correlations between serum modafinil level and neuroimaging data
There were no significant correlations between serum modafinil levels and brain activity changes observed in the amygdala during the FMT, in the PFC during the 2-back task or in the ACC during the VAC task (all p>0.05).
Clinical Variables and Drug Levels
There was no significant effect of modafinil at 100 mg per day either on heart rate or on systolic/diastolic blood pressure (modafinil vs placebo all p>0.2). Serum modafinil level 3 h after administration measured just before the MRI session ranged from 0.2 to 3.38 μg/ml (mean± SD=1.9±1.1 μg/ml). There was a trend for significance for reduced anxiety level (HAM-A) on modafinil (t=1.72, df=32, p=0.095). Subjects also reported decreased fatigue-inertia (t=2.2, df=32, p=0.04) and increased vigor-activity (t=−2.32, df=32, p=0.03), as well as decreased anger-hostility (t=2.01, df=32, p=0.05) on the POMS subscales. These measures, however, did not survive correction for multiple testing.
There was a significant positive correlation between modafinil-induced changes in the anger-hostility subscale and serum modafinil levels (ρ=0.42, df=23, p=0.02) (ie, higher modafinil levels were associated with greater decrement in anger-hostility scores). No other significant correlations were found between serum modafinil levels and changes in other POMS subscales or anxiety levels measured with HAM-A scale (all p>0.05).
DISCUSSION
Our findings show that modafinil in low doses has a seemingly unique profile of effects on cognitive and emotion-processing circuitry in the brain, in contrast to other psychostimulants such as amphetamine. Although amphetamine at low doses tends to increase amygdala reactivity (Hariri et al, 2002), consistent with its anxiogenic effects (Angrist and Gershon, 1970; Ellinwood et al, 1973; Hall et al, 1988), modafinil, in contrast, reduces amygdala response to emotionally salient stimuli. Although not statistically significant, it should be noted that modafinil showed a trend toward significance for reduced anxiety level and hostility, along with an improvement in the subjective energy state. With respect to the effect of modafinil on the PFC during cognitive processing, its effects are similar to amphetamine, with a decrease in PFC activation occurring without a change in performance (Mattay et al, 2003). We will discuss these neuroimaging findings as they relate to the modulatory effects of modafinil on the neurophysiological response during emotional information processing and executive cognition.
Modulatory Effect of Modafinil on the Emotional Information-Processing Circuit
Our results show that modafinil decreases amygdala reactivity to fearful stimuli. These findings are in contrast to other cognition-enhancing psychostimulants. Amphetamine, for example, has a pronounced effect on emotional behavior, including the generation of fear and anxiety (Angrist and Gershon, 1970; Ellinwood et al, 1973; Hall et al, 1988). Moreover, using the FMT, Hariri et al (2002) had previously shown exaggerated amygdala reactivity during the perceptual processing of angry and fearful facial expressions with low-dose amphetamine. In addition to our observation that modafinil induces a diminished amygdala response during the same circumstances, we also found increased negative coupling between the supragenual cingulate and amygdala on modafinil on a functional connectivity analysis.
Although the molecular mechanisms underlying the effect of modafinil on amygdala reactivity is not clear, the increase in negative functional coupling between the supragenual cingulate and the amygdala suggests that top–down control mechanisms may be responsible at the neural systems level for the effects of modafinil in dampening amygdala reactivity and the reduced amygdala reactivity could consequently induce the reduction in its positive coupling with the subgenual ACC. However, because functional connectivity does not give any information regarding directionality, further studies are necessary to confirm this hypothesis. Alternately, as the amygdala is rich in catecholaminergic and serotoninergic projections, it is likely that the decreased amygdalar reactivity on modafinil may be due to alterations in intra-amygdalar signaling resulting from alterations in levels of NE, DA, serotonin, or GABA or from a combination of these effects. As reduction of amygdala reactivity—as observed in our study—has previously been reported after the administration of drugs that act on noradrenergic system as well as with drugs that act on 5HT system (see, eg, reboxetine on noradrenergic system, Norbury et al, 2007; citalopram on serotonergic system, Harmer et al, 2006), and because modafinil acts on both these systems as well as on many other neurotransmitter pathways, it is difficult to define the specific mechanism through which modafinil modulates amygdala activity. It is likely that the more complex action of modafinil on different neurotransmitter pathways compared with amphetamine may be responsible for the differing effect on amygdala reactivity reported with these two different psychostimulant drugs. Further studies are warranted to identify which neurotransmitter systems and interactions that modafinil acts on to modulate amygdala activity.
Modulatory Effects of Modafinil on the Neurophysiological Response During Executive Cognition
Our results show that during the WM and VAC tasks, modafinil decreased BOLD signal in the PFC and ACC, respectively, when compared with placebo, for the same level of task performance. This phenomenon may reflect an improvement in the efficiency of information processing in these regions that are richly innervated by catecholaminergic neurons and is similar to the results of numerous neuroimaging studies in the literature involving catecholaminergic effects (eg, Apud et al, 2007; Cools et al, 2002; Cools and Robbins, 2004; Gibbs and D'Esposito, 2005, 2006; Mattay et al, 2002, 2003; Mehta et al, 2000).
The results on WM are consistent with those reported by Thomas and Kwong (2006) in healthy volunteers without sleep deprivation, albeit after a single 200 mg dose of modafinil. Furthermore, most functional neuroimaging studies have shown an improvement in information processing, within the PFC after modafinil administration. This has been shown in narcolepsy (Saletu et al, 2007), in schizophrenia (Hunter et al, 2006; Spence et al, 2005), and in normal volunteers without sleep deprivation (Minzenberg et al, 2008). Although the nature of the block-design of the 2-back task does not allow the identification of the exact cognitive subprocesses modulated by modafinil (encoding, maintenance, retrieval, or updating), the use of the same paradigm as in previous drug studies permits a comparison of the effect of modafinil with other cognitive-enhancing agents, such as amphetamine and tolcapone, albeit in different participants (Mattay et al, 2003; Apud et al, 2007). Nevertheless, further studies using event-related WM paradigms are necessary to clarify which subprocesses are modulated by the drug. Our results are also in accordance with animal and human behavioral data that showed an improvement with modafinil in performance in several domains of cognition including WM, recognition memory, sustained attention, and cognitive control (Minzenberg and Carter, 2008; Turner et al, 2003; Winder-Rhodes et al, 2010, in press).
The improvement in efficiency of information processing in the ACC during the VAC task are analogous to the results of Blasi et al (2005) who showed an effect of the val158met functional polymorphism in the catechol-o-methyl transferase (COMT) gene, a gene known to modulate cortical DA levels. They showed that individuals homozygous for the met allele and putatively higher cortical DA levels had a more efficient response with decreased activity in the ACC during the VAC task when compared with the other two groups, val/val and val/met.
Although the molecular effects of modafinil are generally regarded to be nonspecific, recent studies showed that modafinil potentiates both NE and DA transmission through the inhibition of DAT (Volkow et al, 2009) and NET (Minzenberg and Carter, 2008). Therefore, it is likely that the neurophysiologic effects of modafinil on the PFC and ACC, both of which are rich in catecholaminergic projections, during executive cognition are most likely driven by DA, NE, or both. Converging evidence shows that monoamines including DA and NE improve the neurophysiological signal to noise ratio. Recent animal studies suggest that NE enhances ‘signals’ through postsynaptic 2A adrenoceptors on PFC dendritic spines, whereas DA decreases ‘noise’ through modest levels of D1 receptor stimulation (Brennan and Arnsten, 2008).
The effects of these molecular processes at the neural system level have also consistently been shown through functional neuroimaging studies. Medications that modulate DA and NE system and enhance catecholamine transmission have been shown to increase efficiency of information processing in PFC circuits (Apud et al, 2007; Cools and Robbins, 2004). Taken together with this previous converging evidence from molecular, behavioral, and neuroimaging studies, the improvement in PFC and ACC information processing induced by modafinil that we observed during executive cognition, is most likely mediated by the potentiating effects of either or both DA and NE transmission through the inhibition of DAT and NET.
Some limitations of the study need to be acknowledged. Although the exclusion of data sets based on our rigorous assessment of image quality resulted in a smaller sample than what we started with, we believe this step is critical to ensure comparable image signal and noise characteristics across drug conditions. Moreover, in our study, we found a significant effect that survived correction for multiple comparisons of modafinil during different task paradigms in spite of reducing the sample size. It should also be noted that the final sample size in our study is comparable to the sample sizes of other modafinil neuroimaging studies in the literature (Hunter et al, 2006; Minzenberg et al, 2008; Saletu et al, 2007; Spence et al, 2005; Thomas and Kwong, 2006). Another potential limitation is that this study did not examine dose-response effects. The absence of any effect of modafinil on performance during the 2-back WM task and VAC task as well its weak effects on behavioral mood scales can most likely be explained by the relatively low dose (100 mg per day) that we used in a small sample of carefully screened healthy volunteers. These results, therefore, cannot be extended to the generally prescribed therapeutic dose (200 mg per day) of modafinil. A within-subject dose-response curve study in a larger sample is warranted to further examine this. However, it should be noted that a low dose of 100 mg in our study that did not effect cognitive performance seemed ideal for our imaging purposes, because matching for performance across drug conditions in fMRI studies is conditio sine qua non to ensure that the observed drug-induced regional BOLD signal difference during the cognitive paradigms was not confounded by a difference in task performance. In addition, while it is not clear if modafinil has a direct effect on vascular reactivity, it is unlikely that our results can be due to such an effect particularly because it would be more global and not explain the task and region-specific effects that we observed. These limitations, together with the potential abuse liability of modafinil at higher therapeutic doses (Volkow et al, 2009), suggest that our results should not be interpreted as a positive endorsement of modafinil as a cognition or mood enhancer. Moreover, the exact mechanism of modafinil effect on amygdala activity cannot be explained with our fMRI data. Further studies to investigate the direct comparison of modafinil with other drugs acting on the circuit underlying anxiety (such as amphetamine, nicotine, benzodiazepines) may be useful to elucidate the neurotransmitter systems implicated in modafinil effects on amygdala.
Notwithstanding some limitations, to our knowledge, this is the first in vivo demonstration in humans of multiple effects of modafinil. Most importantly, modafinil in low doses in the absence of any changes in blood pressure or heart rate, while improving the efficiency of cognitive information processing also seems to dampen reactivity of the amygdala, a brain region implicated in anxiety, to threatening stimuli. The latter may confer an advantage to modafinil over other cognition-enhancing psychostimulants, such as amphetamine, which tend to be anxiogenic with increased liability to abuse and risk of adverse effects on the cardiovascular system.
References
Angrist BM, Gershon S (1970). The phenomenology of experimentally induced amphetamine psychosis—preliminary observations. Biol Psychiatry 2: 95–107.
APA (1994). DSM-IV. Diagnostic and statistical manual. 4th edn, American Psychiatry Association: Washington, DC.
Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R et al (2007). Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology 32: 1011–1020.
Becker PM, Schwartz JR, Feldman NT, Hughes RJ (2004). Effect of modafinil on fatigue, mood, and health-related quality of life in patients with narcolepsy. Psychopharmacology (Berl) 171: 133–139.
Blasi G, Mattay VS, Bertolino A, Elvevåg B, Callicott JH, Das S et al (2005). Effect of catechol-o-methyltransferase val158met genotype on attentional control. J Neurosci 25: 5038–5045.
Brennan AR, Arnsten AF (2008). Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann NY Acad Sci 1129: 236–245.
Broughton RJ, Fleming JA, George CF, Hill JD, Kryger MH, Moldofsky H et al (1997). Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of excessive daytime sleepiness in narcolepsy. Neurology 49: 444–451.
Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B et al (2003). Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry 160: 709–719.
Cools R, Robbins TW (2004). Chemistry of the adaptive mind. Philos Transact A Math Phys Eng Sci 362: 2871–2888.
Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM (2002). Dopaminergic modulation of high-level cognition in Parkinson's disease: the role of the prefrontal cortex revealed by PET. Brain 125: 584–594.
Deroche-Gamonet V, Darnaudéry M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV (2002). Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology (Berl) 161: 387–395.
Ekman P, Friesen WV (1976). Pictures of Facial Affect. Consulting Psychologist Press: Palo Alto, CA.
Ellinwood Jr EH, Sudilovsky A, Nelson LM (1973). Evolving behavior in the clinical and experimental amphetamine (model) psychosis. Am J Psychiatry 130: 1088–1093.
Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM (1999). Stochastic designs in event-related fMRI. Neuroimage 10: 607–619.
Gibbs SE, D'Esposito M (2005). A functional MRI study of the effects of bromocriptine, a dopamine receptor agonist, on component processes of working memory. Psychopharmacology (Berl) 180: 644–653.
Gibbs SE, D'Esposito M (2006). A functional magnetic resonance imaging study of the effects of pergolide, a dopamine receptor agonist, on component processes of working memory. Neuroscience 139: 359–371.
Gorman SH (2002). Determination of modafinil, modafinil acid and modafinil sulfone in human plasma utilizing liquid-liquid extraction and high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 767: 269–276.
Hall RC, Popkin MK, Beresford TP, Hall AK (1988). Amphetamine psychosis: clinical presentations and differential diagnosis. Psychiatr Med 6: 73–79.
Hamilton M (1959). The assessment of anxiety states by rating. Br J Med Psychol 32: 50–55.
Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR (2002). Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology 27: 1036–1040.
Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM (2006). Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59: 816–820.
Hermant JF, Rambert FA, Duteil J (1991). Awakening properties of modafinil: effect on nocturnal activity in monkeys (Macaca mulatta) after acute and repeated administration. Psychopharmacology (Berl) 103: 28–32.
Hunter MD, Ganesan V, Wilkinson ID, Spence SA (2006). Impact of modafinil on prefrontal executive function in schizophrenia. Am J Psychiatry 163: 2184–2186.
Jr JL, Regan C, Stump G, Tannenbaum P, Stevens J, Bone A et al (2009). Hemodynamic and cardiac neurotransmitter- releasing effects in conscious dogs of attention- and wake-promoting agents: a comparison of d-amphetamine, atomoxetine, modafinil, and a novel quinazolinone H3 inverse agonist. J Cardiovasc Pharmacol 53: 52–59.
MacDonald JR, Hill JD, Tarnopolsky MA (2002). Modafinil reduces excessive somnolence and enhances mood in patients with myotonic dystrophy. Neurology 59: 1876–1880.
Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L et al (2006). Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther 319: 561–569.
Makris AP, Rush CR, Frederich RC, Kelly TH (2004). Wake-promoting agents with different mechanisms of action: comparison of effects of modafinil and amphetamine on food intake and cardiovascular activity. Appetite 42: 185–195.
Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF et al (2003). Catechol o-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA 100: 6186–6191.
Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN et al (2002). Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol 51: 156–164.
McNair DM, Lorr M, Droppleman LF (1992). Revised Manual for the Profile of Mood States. Educational and Industrial Testing Service: San Diego, CA.
Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW (2000). Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci 20: RC65.
Minzenberg MJ, Carter CS (2008). Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology 33: 1477–1502.
Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS (2008). Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science 322: 1700–1702.
Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ (2007). Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. Br J Psychiatry 190: 531–532.
Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS et al (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8: 828–834.
Provigil website. http://www.provigil.com/media/PDFs/prescribing_info.pdf Accessed 29 March 29 2010 2010.
Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y (2008). Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci 28: 8462–8469.
Robertson Jr P, Hellriegel ET (2003). Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet 42: 123–137.
Saletu M, Anderer P, Semlitsch HV, Saletu-Zyhlarz GM, Mandl M, Zeitlhofer J et al (2007). Low-resolution brain electromagnetic tomography (LORETA) identifies brain regions linked to psychometric performance under modafinil in narcolepsy. Psychiatry Res 154: 69–84.
Samuels ER, Hou RH, Langley RW, Szabadi E, Bradshaw CM (2006). Comparison of pramipexole and modafinil on arousal, autonomic, and endocrine functions in healthy volunteers. J Psychopharmacol 20: 756–770.
Schwartz JR, Hirshkowitz M, Erman MK, Schmidt-Nowara W (2003). Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea: a 12-week, open-label study. Chest 124: 2192–2199.
Simon P, Panissaud C, Costentin J (1994). The stimulant effect of modafinil on wakefulness is not associated with an increase in anxiety in mice. A comparison with dexamphetamine. Psychopharmacology (Berl) 114: 597–600.
Spence SA, Green RD, Wilkinson ID, Hunter MD (2005). Modafinil modulates anterior cingulate function in chronic schizophrenia. Br J Psychiatry 187: 55–61.
Tan HY, Callicott JH, Weinberger DR (2007). Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex 17 (Suppl 1): i171–i181.
Taneja I, Haman K, Shelton RC, Robertson D (2007). A randomized, double-blind, crossover trial of modafinil on mood. J Clin Psychopharmacol 27: 76–79.
Thomas RJ, Kwong K (2006). Modafinil activates cortical and subcortical sites in the sleep-deprived state. Sleep 29: 1471–1481.
Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ (2003). Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl) 165: 260–269.
van Vliet SA, Jongsma MJ, Vanwersch RA, Olivier B, Philippens IH (2006). Behavioral effects of modafinil in marmoset monkeys. Psychopharmacology (Berl) 185: 433–440.
Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F et al (2009). Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA 301: 1148–1154.
Wesensten NJ (2006). Effects of modafinil on cognitive performance and alertness during sleep deprivation. Curr Pharm Des 12: 2457–2471.
Winder-Rhodes S, Chamberlain S, Idris M, Robbins T, Sahakian B, Müller U (2010). Effects of modafinil and prazosin on cognitive and physiological functions in healthy volunteers. J Psychopharmacol, print copy in press (originally published online 3 Jun 2009, at http://jop.sagepub.com/cgi/rapidpdf/0269881109105899v1).
Zifko UA, Rupp M, Schwarz S, Zipko HT, Maida EM (2002). Modafinil in treatment of fatigue in multiple sclerosis. Results of an open-label study. J Neurol 249: 983–987.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health. We thank Saumitra Das, MA, Guilna Alce, BS, Ajay Premkumar, Alan Lazerow, BA, and Natkai Akbar, BS, for their invaluable research assistance. We thank Steven H Gorman, BS, and Alexander Kogan, BS, Cephalon, Inc., for information related to measurement of plasma modafinil levels, and Cephalon, Inc., for the measurement of plasma modafinil levels.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr TE Goldberg is consultant for MERK and GSK. All other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Rasetti, R., Mattay, V., Stankevich, B. et al. Modulatory Effects of Modafinil on Neural Circuits Regulating Emotion and Cognition. Neuropsychopharmacol 35, 2101–2109 (2010). https://doi.org/10.1038/npp.2010.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2010.83
Keywords
This article is cited by
-
Modafinil Improves Autism-like Behavior in Rats by Reducing Neuroinflammation
Journal of Neuroimmune Pharmacology (2023)
-
Modafinil enhances cognitive, but not emotional conflict processing via enhanced inferior frontal gyrus activation and its communication with the dorsomedial prefrontal cortex
Neuropsychopharmacology (2020)
-
Wake-Promoting and EEG Spectral Effects of Modafinil After Acute or Chronic Administration in the R6/2 Mouse Model of Huntington's Disease
Neurotherapeutics (2020)
-
Moral decision making under modafinil: a randomized placebo-controlled double-blind crossover fMRI study
Psychopharmacology (2019)
-
MAM-E17 rat model impairments on a novel continuous performance task: effects of potential cognitive enhancing drugs
Psychopharmacology (2017)