Abstract
Measuring the in vivo occupancy of antipsychotic drugs at dopamine D2 and D3 receptors separately has been difficult because of the lack of selective radiotracers. The recently developed [11C]-(+)-PHNO is D3-preferring, allowing estimates of the relative D2 and D3 binding of antipsychotic drugs. We used positron emission tomography (PET) imaging in baboons with [11C]-(+)-PHNO to examine the binding of clozapine and haloperidol to D2 and D3 receptors. Four animals were scanned with dynamically acquired PET and arterial plasma input functions. Test and retest scans were acquired in single scanning sessions for three subjects to assess the reproducibility of [11C]-(+)-PHNO scans. Four additional scans were acquired in each of three subjects following single doses of antipsychotic drugs (clozapine 0.5534 mg/kg, haloperidol 0.0109 mg/kg, two administrations per drug per subject) and compared with baseline scans. The percent change in binding (ΔBPND) following challenges with antipsychotic drugs was measured. A regression model, based on published values of regional D2 and D3 fractions of [11C]-(+)-PHNO BPND in six brain regions, was used to infer occupancy at D2 and D3 receptors. BPND following antipsychotic challenge decreased in all regions. Estimated D2 : D3 selectivity was 2.38 for haloperidol and 5.25 for clozapine, similar to published in vitro values for haloperidol (3.03), but slightly higher for clozapine (2.82). These data suggest that acute doses of clozapine and haloperidol bind to D3 receptors in vivo, and that the lack of D3 occupancy by antipsychotics observed in some recent imaging studies may be because of other phenomena.
Similar content being viewed by others
INTRODUCTION
Historically, research into the dopaminergic aspects of the mechanism of function of antipsychotic medications has focused on antagonism of the dopamine-2 (D2) receptor (Creese et al, 1976; Seeman and Lee, 1975). Dopamine-3 (D3) receptors belong to the same class of receptors as D2, and there is evidence they may have a role in several neuropsychiatric disorders, including addiction, Parkinson's disease, and schizophrenia (Sokoloff et al, 2006). In particular, therapeutic agents targeted towards the D3 receptor may hold promise as novel antipsychotic agents. Until recently, direct in vivo evidence of D3 receptor dysfunction in neuropsychiatric populations has not been possible to obtain because of the lack of a positron emission tomography (PET) or single photon emission computed tomography (SPECT) imaging agent that can suitably distinguish between dopamine D3 and D2 binding. Hence, the potential contribution of D3 receptor blockade to antipsychotic efficacy has been difficult to assess.
[11C]-(+)-PHNO is a recently developed dopamine D2/3 radioligand (Wilson et al, 2005) that is strongly D3 preferring (Narendran et al, 2006). In vivo imaging in non-human primates and mouse autoradiography paradigms have demonstrated that PHNO binding in the midbrain dopamine nuclei (substantia nigra, ventral tegmental area, SN/VTA) is almost completely due to D3 binding (Rabiner et al, 2009). [11C]-(+)-PHNO binding also displays vulnerability to competition by selective D3 agents in other D3-rich brain regions such as globus pallidus (GP), and ventral striatum (VST) (Narendran et al, 2006; Rabiner et al, 2009; Searle et al, 2010). These findings suggest that the D3-preferring ligand [11C]-(+)-PHNO can be used to ascertain the extent of D3 binding by antipsychotics.
Extensive preclinical data suggest that many antipsychotic medications bind to D2 receptors with moderately higher affinity than they do to D3 receptors (Table 1) (Levant, 1997; Schotte et al, 1996). On the basis of these in vitro selectivity ratios, predicted occupancy of D3 at therapeutic doses would be lower than D2 occupancy, but still non-negligible (Table 1). However, D3-binding levels by antipsychotics were called into question by a recent study by Graff-Guerrero et al (2009), which used [11C]-(+)-PHNO to investigate in vivo binding of antipsychotics to D3. Patients with schizophrenia on stable, chronically-administered doses of clozapine (CLZ), risperidone, or olanzapine showed no decrease or even increased [11C]-(+)-PHNO binding in GP and modest decrease in VST (17±35%) when compared with 20 healthy control subjects at baseline, whereas binding in the D2-dominated dorsal striatum was considerably reduced compared with controls. Specific binding of the D2-preferring tracer [11C] raclopride, which in VST and GP is predominantly because of D2, was substantially reduced in all measured brain regions in patients compared with controls. These results suggested either that antipsychotics do not bind D3 receptors in vivo, there is a change in the availability of D3 receptors following chronic administration of drugs (that is, upregulation) or a change in the affinity of [11C]-(+)-PHNO to D3 receptors in schizophrenia. Another study from the same research group used ex vivo and in vitro [3H]-(+)-PHNO binding in rat brain and found that acute doses of CLZ, olanzapine, haloperidol (HAL), and risperidone demonstrated lower selectivity for the D3 compared with D2 receptors ex vivo than in vitro (McCormick et al, 2010).
Determining the degree of antipsychotic binding to the D3 receptor, if any, has important implications for the development of novel treatments for schizophrenia. Given the discrepancies between the older in vitro literature and recent ex and in vivo reports about the binding of antipsychotic medications to the D3 receptor and the importance of identifying whether or not, and to what degree, antipsychotic medications bind to D3 receptors, we undertook to examine the acute binding of antipsychotics to D3 receptors in the in vivo setting. We also had concern, however, that because of the much higher affinity of [11C]-(+)-PHNO for D3 compared with D2 receptors (Beaver et al, 2009), there was a potential for our results to be confounded by mass-carryover effects at D3, but not D2, receptors. Therefore, we conducted this study in two phases. First, we performed test-retest scans in three anesthetized baboons to ascertain if there would be mass-carryover effects when two [11C]-(+)-PHNO scans were conducted in a single scanning session. Next, we performed an acute dose study in anesthetized baboons with two commonly used and prototypical antipsychotic agents, CLZ, and HAL. We measured the binding of [11C]-(+)-PHNO in subcortical brain regions before and after administration of CLZ and HAL. We then estimated individual D2 and D3 receptor occupancies, as well as the D2 : D3 receptor selectivity of these medications using a model based on all of the data.
SUBJECTS AND METHODS
Subjects
Three adult male baboons were scanned in the test-retest study (baboons A, B, and C, papio Anubis, 20.1±4.8 kg) and antipsychotic studies (baboons B, C, and D, papio anubis, 23.9±2.4 kg; Table 2). A minimum of 12 days was allowed between each experimental day for each baboon and no baboon was studied more than three times per month. Study procedures were approved by the Institutional Animal Care and Use Committees (IACUC) of Columbia University and the New York State Psychiatric Institute.
Test-Retest Study
To detect a potential mass-carryover effect at the D3 site, three baboons were scanned at baseline and then 172±36 min later (retest condition 52±36 min after the end of the test scan; Table 2). The percent change in binding potential (see Data Analysis, below), ΔBPND, was used as an outcome measure to assess reproducibility. This signed formulation was used rather than a measure of variability because the direction of change across conditions was the quantity of interest.
Antipsychotic Study
Baseline [11C]-(+)-PHNO scans were acquired for each subject. Two baboons underwent three baseline scans and one baboon underwent two baseline scans (Table 2). The average of each subject's baseline data was used for comparison with the drug challenge data. For challenge scans, each baboon received CLZ (0.5534 mg/kg) or HAL (0.0109 mg/kg), dissolved in 10 ml of saline vehicle. These doses were selected to attain target occupancies of 50% (CLZ) or 75% (HAL) in dorsal striatum where binding is predominantly to the D2 receptors. Challenges were administered manually as a five-minute intravenous (iv) bolus, 15 min before the scan. Each subject underwent two scans following each drug. In all, 20 scans were acquired (eight baseline scans plus three subjects * two drugs * two post-challenge scans per drug).
Radiochemistry
[11C]-(+)-PHNO was prepared as described by Wilson et al (2005), with some modifications (Rabiner et al, 2009).
PET Procedures
Fasted animals were initially immobilized with ketamine (10 mg per kg i.m.). Anesthesia during preparation and scans was maintained with 2% isoflurane via endotracheal tube. An iv catheter was inserted for radioligand administration, drug administration and hydration. An arterial catheter was placed in a femoral artery for arterial blood sampling and continuous blood pressure monitoring. Vital signs (blood pressure, pulse, EKG, temperature, pulse oximetry, and respiration) were monitored continuously using a patient monitoring system (DataScope Corp, Paramus, NJ) and remained within normal parameter ranges during all studies that did not include the administration of CLZ. Subjects' blood pressures did decrease by ∼20% during administration of CLZ. In all cases, hemodynamic parameters normalized with gentle administration of normal saline. No subject demonstrated other abnormalities in vital signs or EKG or required resuscitative measures. Temperature was maintained at 37°C with a heated water blanket. All scanning was performed on an HR+ scanner (Siemens, Knoxville, TN), operating in 3D mode. The animal's head was placed in the center of the field of view. A 10-min transmission scan was acquired before tracer injection for attenuation correction. [11C]-(+)-PHNO was administered as a 30 s iv bolus. Emission data were binned into a sequence of frames of increasing length. Each scan included 22 frames (4 × 15 s, 2 × 1 min, 4 × 2 min, 2 × 5 min, and 10 × 10 min). Total scan durations were 121 min. PET data were corrected for attenuation, scatter, and randoms and reconstructed using filtered back-projection with a Shepp filter (cutoff 0.5 cycles/ ray).
Arterial plasma samples were collected using an automated sampling system during the first 4 min (11 samples), and manually thereafter at longer intervals. A total of 22 arterial samples were collected for input function measurement. Six additional samples were collected during each scan for HPLC analysis of the unmetabolized fraction of radiotracer. Aliquots were also removed from six of the obtained samples for analysis of CLZ and HAL plasma levels.
Data Analysis
Plasma data
Arterial plasma input functions were formed as described previously (Abi-Dargham et al, 2000). Briefly, centrifugation was performed to separate plasma from whole blood and activity in plasma was counted on a gamma counter (Wallac 1480, Perkin-Elmer, Boston MA). The unmetabolized fraction of radioligand was measured in the six samples taken for this analysis by HPLC using a fraction collection method. The measured fractions were fitted to a sum of two exponential functions. Whole-blood activity was multiplied by this parent-fraction curve to obtain an empirical estimate of the unmetabolized radiotracer concentration in arterial plasma. The empirical curve was fitted to a sum of three exponentials starting at the time of peak concentration. This modeled curve was used as the input function for compartment analysis. Peripheral clearance of radiotracer (L/hr) was computed as the injected activity divided by the area under the curve of the input function. Additionally, fp, the fraction of unmetabolized radiotracer not bound to plasma proteins, was measured with an ultrafiltration technique as previously described (Ekelund et al, 2007). For fp measurement, a small quantity of [11C]-(+)-PHNO was added to a sample of arterial plasma collected before radiotracer administration.
PET data
Decay-corrected reconstructed data were co-registered to T1 weighted anatomical MRIs acquired for each animal, using maximization of mutual information as implemented in the SPM2 software environment (Friston et al, 1995). Regions of interest (ROIs) were drawn on the MRIs and transferred to the co-registered PET images. ROIs included putamen (PUT), caudate (CAU), VST, GP, SN/VTA, and thalamus (THA). Time activity curves were generated from the mean activity in each region and each frame. Data were fitted using a two-tissue compartment model (2TC) with arterial plasma input, with cerebellum as reference region. The total distribution volume (VT) was computed in each region including cerebellum, and BPND, the binding potential relative to the non-displaceable compartment (Innis et al, 2007), was computed as:

For the antipsychotic study, combined D2 and D3 receptor occupancy by CLZ and HAL was estimated as −ΔBPND, where ΔBPND is the percent change in BPND across conditions in each ROI,

To estimate the D2 : D3 selectivity of CLZ and HAL, data from all ROIs were fitted to a linear model that used previously established regional fractions of the D2 and D3 contributions to total [11C]-(+)-PHNO BPND in anesthetized baboons in each of the regions:

where fD3 and fD2 are the fractions of [11C]-(+)-PHNO BPND attributable to each receptor type (Table 3) (Rabiner et al, 2009), occ(*) is the drug occupancy at each receptor type. The selectivity for D2 vs D3 was then computed from the estimated occupancies as

where ED50(*) is the estimated dose at which 50% of receptor type * is occupied by drug. Equation (4) follows from the rearrangement of the equilibrium mass action law for reversibly binding ligands (Equation (5))

RESULTS
Test-Retest Study
The scan parameters for the test-retest study are shown in Table 4. There were no significant differences between the baseline and retest conditions on any scan parameter. Regional BPND and ΔBPND values are presented in Table 5. One test value in GP was excluded because of unstable parameter estimates. The mean retest BPND was lower than the test BPND by 20% or more in all D3-rich regions (GP, VST, THA, SN/VTA), reaching statistical significance in VST, but was within 7% of test BPND in dorsal striatum (PUT and CAU). These results indicated that in D3-rich regions, [11C]-(+)-PHNO binding potential decreased purely from an order effect, suggesting the possibility that use of pre- and post- antipsychotic challenge scans from the same scanning session would introduce a quantification artifact. Therefore, in the subsequent antipsychotic occupancy study, only one antipsychotic challenge scan was acquired per session without baseline, thereby avoiding any possible mass carryover or other order effect from scanning twice in the same session. To measure the change across conditions, BPND obtained from each antipsychotic challenge scan was individually compared with an average of previous baseline scans for the same subject, maintaining the within-subject design of this study.
Antipsychotic Study
The scan parameters for the Antipsychotic Study are shown in Table 6. There were no significant differences on any scan parameter across all conditions.
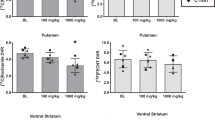
Plasma input functions and time activity curves from several brain regions, averaged across all scans before and after administration of 0.5534 mg/kg of CLZ and 0.0109 mg/kg of HAL, are shown in Figure 1a and b. Representative BPND maps from one subject across all conditions are shown in Figure 2. Regional BPND values at baseline and after administration of CLZ and HAL are shown in Table 7. Both CLZ and HAL decreased [11C]-(+)-PHNO to an extent that was close to the target level in the dorsal striatum (44% decrease for CLZ, 70% decrease for HAL, Table 7).
(a) Averaged arterial plasma concentration of [11C]-(+)-PHNO under baseline (▾, n=8 scans), clozapine challenge (▴, n=6 scans) and haloperidol challenge (•, n=6 scans) conditions. Continuous curves (—) represent the average of the modeled fits as described in the Methods section. Data are normalized to injected activity. The graph shows that [11C]-(+)-PHNO concentration in arterial plasma was unaffected by the drug challenges. (b) Averaged time activity curves from all studies under baseline (▾, n=8 scans), clozapine challenge (▴, n=6 scans) and haloperidol challenge (•, n=6 scans) conditions. Continuous curves (—) represent the average of the two-tissue compartment model (2TC) fits. Error bars are the SD across the measured data. Data are normalized to injected activity and body mass and are shown for a region with similar contributions from dopamine-2 (D2) and dopamine-3 (D3) binding (globus pallidus, (GP)), mostly D2 binding (putamen) and mostly D3 binding (substantia nigra/ventral tegmental area (SN/VTA)), as well as the cerebellum.
[11C]-(+)-PHNO BPND maps in one baboon at the level of the GP across the three conditions. Images are the average of three baseline scans and two post-drug scans for each antipsychotic, and were generated at each voxel with the SRTM algorithm.
Regression Model-Based Estimates for D2 : D3 Selectivity and Occupancy
Using the model from Equation (3) and the in vivo data acquired in this study, CLZ in vivo occupancy (±95% confidence interval) was 47% (±14%) at D2 and 15% (±12%) at D3. HAL in vivo occupancy was 76% (±18%) at D2 and 57% (±14%) at D3. ED50 values were computed based on the administered doses and these occupancy estimates. ED50 values for CLZ were 0.6135 mg/kg at D2 and 3.2216 mg/kg at D3. HAL ED50 values were 0.0035 mg/kg at D2 and 0.0083 mg/kg at D3.
The D2 : D3 selectivities of CLZ and HAL were calculated, based on these occupancy estimates. In vivo, HAL selectivity was very similar to in vitro predictions (2.38 in vivo compared with 3.03±4.42, n=11 in vitro studies). In vivo selectivity for CLZ was approximately one SD higher than the mean of nine in vitro studies (5.25 in vivo compared with 2.82±2.01 in vitro).
DISCUSSION
This study used [11C]-(+)-PHNO to investigate whether acute doses of the antipsychotic medications CLZ and HAL bind in vivo to the D3 receptor and to estimate their D2 : D3 receptor selectivities. We observed that acute doses of HAL bind to D3 receptors within the range predicted by in vitro data, and that CLZ does bind to D3 receptors at the lower end of the range predicted by in vitro data. ΔBPND in dorsal striatum (PUT, CAU), which is attributable mostly to D2 occupancy, was similar to the clinical range for both drugs (that is, ∼45% for CLZ and ∼70% for HAL). ΔBPND was lower in D3-rich regions, but measurable, as most clearly illustrated in the SN/VTA region, where [11C]-(+)-PHNO specific binding is almost exclusively due to D3 receptor binding (Narendran et al, 2006; Rabiner et al, 2009; Searle et al, 2010). Our results differ from those of Graff-Guerrero et al and McCormick et al in some important ways. We observed decreased binding of [11C]-(+)-PHNO in GP following CLZ, consistent with D3 binding by CLZ, whereas Graff-Guerrero et al observed no decrease (relative to matched controls) or even increased [11C]-(+)-PHNO binding in GP. We observed robust binding of HAL to D3, whereas McCormick et al observed negligible binding of HAL to D3 when administered in vivo and measured ex vivo in rats.
Graff-Guerrero et al reported that chronically administered olanzapine, risperidone, and CLZ in patients with schizophrenia did not occupy D3, and that in some cases paradoxical increases in radioligand binding were observed (Graff-Guerrero et al, 2009). The data we report in this study show that both CLZ and HAL do bind to D3 receptors when administered acutely to anesthetized primates; this suggests that the observation in patients could be attributable to some other phenomenon such as upregulation of receptors following chronic administration, as discussed by the authors. The regulation of D3 expression has been shown to be distinct from that of D2 in a number of ways (Sokoloff et al, 2006), and currently there is insufficient evidence either corroborating or ruling out the upregulation scenario. Another difference between the studies is the use of anesthesia in this study. Although it has been shown that isoflurane anesthesia can cause alterations in PET measurements of stimulant-induced dopamine release (Tsukada et al, 2002), we are not aware of studies that have examined the effect of anesthesia on PET measurements of receptor occupancy by antipsychotic drugs, but this remains a possibility we cannot rule out. Additionally, our selectivity estimate used the assumption that the fractions of [11C]-(+)-PHNO binding to D2 and D3 receptors in this cohort were similar to those measured in our previous studies with D3-selective agents. Although we have found these parameters to be consistent across subjects, we did not measure them directly in this study.
However, there are other methodological differences between the studies that could offer a simpler explanation for the differing conclusions. We used arterial plasma-based kinetic models following 120 min of emission data, while in the Graff–Guerrero study the authors used reference region analysis (SRTM, Lammertsma and Hume, 1996) following 90 min of data acquisition. The arterial-based method is a direct implementation of the equations of the 2TC model, whereas the SRTM algorithm is derived from the 2TC equations with additional simplifying assumptions. Although these two methods give correlated estimates of BPND for many ligands, they are not identical, and can be quite different in cases where the data do not conform well to the simplifying assumptions of SRTM (Slifstein et al, 2000). We measured modest decreases in GP [11C]-(+)-PHNO binding following CLZ (21±14%) that still, however, included a component of binding of CLZ to D3. It is possible that these relatively modest decreases were difficult to detect with shorter scans and SRTM in this brain region where [11C]-(+)-PHNO has complex kinetics, and especially slow washout. To test this, we reexamined our data under three additional conditions: the same analysis as before with data truncated to 90 min, and SRTM with both 90 and 120-min data. With the plasma-based approach, baseline binding measures were nearly identical at both durations in the D2-dominated dorsal striatum (90 min BPND=100±5% of 120 min BPND in caudate and 103±2% of 120 min BPND in putamen), whereas in the D3-rich regions the binding measures tended to be decreased following truncation (90 min BPND=94±8% of 120 min BPND in GP, 92±7% in VST, 91±4% in thalamus, 93±4% in SN/VTA). With 120 min SRTM BPND, the ratio to plasma-based BPND decreased as a function of binding magnitude (Figure 3), and this effect was even more pronounced with 90-min SRTM BPND (Figure 3). When the occupancy model was applied to 120-min SRTM data, D2 : D3 selectivity was 3.6 for HAL but increased to 19 for CLZ. When applied to 90-min data, selectivity was 3.7 for HAL and 49 (that is, nearly undetectable) for CLZ. Thus in these data at least, there was a pronounced qualitative difference in outcome associated with both scan duration and method of analysis, suggesting the longer duration with 2TC may be a more sensitive and reliable approach in this setting. It remains to be seen if a similar relationship is observable in data acquired in human subjects; our data in this study highlight the importance of comparing the different approaches in that setting.
Scatter plots of regions of interest (ROI) analysis using SRTM. The left panel shows SRTM-derived BPND using the full 120 min of data plotted against 2TC-derived BPND. The graph shows that there is a similar proportionality between methods across moderate-binding regions, but not in high-binding regions. In the right panel, SRTM-derived data using 90-min truncated data are plotted against SRTM-derived data using 120 min of data. The graph shows the measures are virtually identical in the moderate-binding regions, but that the decrease in high-binding regions (relative to 2TC) is more pronounced with the truncated data. The solid line in each graph is the line of identity.
In rat brains, McCormick et al observed that CLZ, HAL, olanzapine, and risperidone all competed robustly with [3H]-(+)-PHNO binding in homogenates from a number of brain regions including the D3-rich cerebellar lobes 9 and 10 and the ventral pallidum, but were much less competitive in the setting of ex vivo autoradiographic measurements of [3H]-(+)-PHNO binding in rats treated with the same drugs and then killed at 1 h after radioligand injection (McCormick et al, 2010). In particular, it was observed that average HAL occupancy in the dorsal striatum (mostly attributable to D2) was 80%, whereas occupancy in cerebellar lobes 9 and 10 (mostly D3) was negligible, in contrast to our data where HAL exhibited robust D3 binding (D2 : D3 selectivity ratio =2.38). The CLZ results from the two studies, on the other hand, are much more consistent with each other.
In addition to species differences, the kinetic methods used in the two studies were different; the ex vivo occupancy estimates in the rodent study were based on tissue ratios of [3H]-(+)-PHNO concentration measured at the single time point of killing across all regions, whereas the primate study used 120 min of dynamic data from each brain region. Additionally, the doses of PHNO were different between the studies. In their ex vivo study, McCormick et al injected 2 nmol of [3H]-(+)-PHNO, approximately 1.8 μg/kg in a 275 g rat. In this study, we injected ∼0.04 μg/kg of [11C]-(+)-PHNO in baboons. The characterization of the precise limits on tracer dose for (+)-PHNO has not been definitively established, but this 45-fold difference in injected concentration suggests that the present data were less likely to have been affected by mass-dose effects, especially at D3 receptors where (+)-PHNO affinity is 20-fold higher than at D2 (Beaver et al, 2009). These facts, however, seem unlikely by themselves to explain why no binding by the high affinity drug HAL was observed in the D3-dominated cerebellar lobes, whereas the lower affinity drug CLZ displayed moderate binding in those regions in rodents.
In this study, the D2 : D3 selectivity ratio for CLZ was slightly higher than that predicted by in vitro literature. One possible explanation for the lower than expected in vivo D3 binding of CLZ observed here following a single iv dose may be a kinetic effect related to its fast off-rate (Seeman, 2002). Kapur and Seeman measured in vitro on and off rates separately for a large number of antipsychotics in homogenates prepared from rat striatum and observed that there was much greater variability in koff than kon between drugs, and that koff therefore accounted for most of the observed affinity differences between drugs (Kapur and Seeman, 2000). The ratio of CLZ kon to HAL kon was 1.4, whereas that of CLZ koff to HAL koff was 81, accounting for most of their affinity difference. These differences, and the fact that plasma clearance of CLZ and HAL following iv injection are similar (Cheng et al, 1988; Kudo and Ishizaki, 1999), suggest that, following a bolus administration, CLZ will dissociate from receptors in response to decreasing plasma concentration much more rapidly than HAL. Receptor occupancy measured during a radiotracer scan (combined D2 and D3 occupancy in this case) is a weighted average of the occupancy over the course of the scan with weight at time t proportional to the concentration of the non-displaceable component (free plus nonspecifically bound) of the radioligand concentration (Endres and Carson, 1998; Kegeles et al, 2008). Compared with dorsal striatum, the slower washout of [11C]-(+)-PHNO from D3-rich regions, especially in the GP, might have caused the measured occupancy to be weighted more towards later times in the scan when CLZ occupancy may have decreased. This effect would be less pronounced for HAL because of its higher affinity and slower off-rate. The presence of this effect in these data is conjectural, but could be tested by administration of CLZ as a constant infusion rather than as a bolus.
In conclusion, we observed that acute doses of both CLZ and HAL bind to D3 receptors in vivo. HAL binds to D3 receptors within the range predicted by in vitro data and CLZ binds to D3 receptors at the lower end of the range predicted by in vitro data. These results suggest that antipsychotic medications do bind to D3 receptors at predicted levels when administered acutely in vivo, and that therefore the apparent lack of occupancy by antipsychotics, including CLZ, in D3-rich regions when administered chronically to patients, is due to some other phenomenon. We have highlighted some of the methodological issues that might have lead to discrepancies in estimates of occupancy in D3-rich regions between our report and previous reports. An alternative explanation that could reconcile our data and those of Graff–Guerrero et al would be the upregulation of D3 receptors following chronic antipsychotic treatment. The regulation of D3 expression has been shown to be distinct from that of D2 in a number of ways (Sokoloff et al, 2006), and additional studies are required to test the upregulation scenario. Future studies should address the effects of chronic administration on expression of D3 and also assess the binding of other commonly used antipsychotic medications to D3 receptors. This information will allow researchers a more complete understanding of the role of the D3 receptor in the pathophysiology and treatment of schizophrenia, and therefore has great implications for the targeted development of novel therapeutics.
References
Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M et al (2000). Measurement of striatal and extrastriatal dopamine D-1 receptor binding potential with [C-11]NNC 112 in humans: validation and reproducibility. J Cerebr Blood F Met 20: 225–243.
Beaver JD, Gallezot JD, Nabulsi N, Weinzimmer D, Singhal T, Slifstein M et al (2009). PHNO in vivo affinity for D2 and D3 receptors: a PET study in rhesus monkeys. J Cerebr Blood F Met 29: S59–S62.
Cheng YF, Lundberg T, Bondesson U, Lindstrom L, Gabrielsson J (1988). Clinical pharmacokinetics of clozapine in chronic schizophrenic patients. Eur J Clin Pharmacol 34: 445–449.
Creese I, Burt DR, Snyder SH (1976). Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192: 481–483.
Ekelund J, Slifstein M, Narendran R, Guillin O, Belani H, Guo NN et al (2007). In vivo DA D-1 receptor selectivity of NNC 112 and SCH 23390. Mol Imaging and Biol 9: 117–125.
Endres CJ, Carson RE (1998). Assessment of dynamic neurotransmitter changes with bolus or infusion delivery of neuroreceptor ligands. J Cereb Blood Flow Metab 18: 1196–1210.
Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frakowiak RSJ (1995). Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping 2: 189–210.
Graff-Guerrero A, Mamo D, Shammi CM, Mizrahi R, Marcon H, Barsoum P et al (2009). The effect of antipsychotics on the high-affinity state of D2 and D3 receptors: a positron emission tomography study With [11C]-(+)-PHNO. Arch Gen Psychiatry 66: 606–615.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Giedde A, Gunn RN et al (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cerebr Blood F Met 27: 1533–1539.
Kapur S, Seeman P (2000). Antipsychotic agents differ in how fast they come off the dopamine D2 receptors. Implications for atypcial antipsychotic action. J Psychiatry Neurosci 25: 161–166.
Kegeles LS, Slifstein M, Frankle WG, Xu X, Hackett E, Bae SA et al (2008). Dose-occupancy study of striatal and extrastriatal dopamine D2 receptors by aripiprazole in schizophrenia with PET and [18F]fallypride. Neuropsychopharmacology 33: 3111–3125.
Kudo S, Ishizaki T (1999). Pharmacokinetics of haloperidol. Clin Pharmacokin 37: 435–456.
Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. Neuroimage 4 (3 Part 1): 153–158.
Levant B (1997). The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev 49: 231–252.
McCormick PN, Kapur S, Graff-Guerrero A, Raymond R, Nobrega JN, Wilson AA (2010). The antipsychotics olanzapine, risperidone, clozapine, and haloperidol are D2-selective ex vivo but not in vitro. Neuropsychopharmacology 35: 1826–1835.
Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E et al (2006). Dopamine (D2/ 3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse 60: 485–495.
Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R et al (2009). In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: studies in non-human primates and transgenic mice. Synapse 63: 782–793.
Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS et al (1996). Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 124: 57–73.
Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M et al (2010). Imaging dopamine D(3) receptors in the human brain with positron emission tomography, [(11)C]PHNO, and a selective D(3) receptor antagonist. Biol Psychiatry 68: 392–399.
Seeman P (2002). Atypical antipsychotics: mechanism of action. Can J Psychiatry 47: 27–38.
Seeman P, Lee T (1975). Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 188: 1217–1219.
Slifstein M, Parsey R, Laruelle M (2000). Derivation of [11C]WAY-100635 binding parameters with reference tissue models: effect of violations of model assumptions. Nucl Med Bio 27: 487–492.
Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E et al (2006). The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets 5: 25–43.
Tsukada H, Miyasato K, Kakiuchi T, Nishiyama S, Harada N, Domino EF (2002). Comparative effects of methamphetamine and nicotine on the striatal [C-11]raclopride binding in unanesthetized monkeys. Synapse 45: 207–212.
Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D et al (2005). Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9 -ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem 48: 4153–4160.
Acknowledgements
This study was funded by the Lieber Center for Schizophrenia Research and partly by GlaxoSmithKline. The authors gratefully acknowledge the expert technical contributions of Elizabeth Hackett, John Castrillon and Sung A Bae.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
RRG has received research support from Janssen and Lilly through APIRE and a travel stipend from Lilly, Forest, and Elsevier Science through the Society of Biological Psychiatry. RNG and EAR are employees of GlaxoSmithKline. In the last 3 years, AA has received honoraria from BMS-Otsuka, Boehringer-Ingelheim, Lundbeck, and Sanofi-Aventis, as well as research support from GlaxoSmithKline. In the last 3 years, MS has served as a consultant for Amgen, and GlaxoSmithKline, and has received research support from Intracellular Therapies, XX, NM, and BE have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Girgis, R., Xu, X., Miyake, N. et al. In Vivo Binding of Antipsychotics to D3 and D2 Receptors: A PET Study in Baboons with [11C]-(+)-PHNO. Neuropsychopharmacol 36, 887–895 (2011). https://doi.org/10.1038/npp.2010.228
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2010.228
Keywords
This article is cited by
-
Imaging synaptic dopamine availability in individuals at clinical high-risk for psychosis: a [11C]-(+)-PHNO PET with methylphenidate challenge study
Molecular Psychiatry (2021)
-
A positron emission tomography occupancy study of brexpiprazole at dopamine D2 and D3 and serotonin 5-HT1A and 5-HT2A receptors, and serotonin reuptake transporters in subjects with schizophrenia
Neuropsychopharmacology (2020)
-
Occupancy of Dopamine D3 and D2 Receptors by Buspirone: A [11C]-(+)-PHNO PET Study in Humans
Neuropsychopharmacology (2016)
-
Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [11C]-(+)-PHNO
Psychopharmacology (2016)
-
Lack of Age-Dependent Decrease in Dopamine D3 Receptor Availability: A [11C]-(+)-PHNO and [11C]-Raclopride Positron Emission Tomography Study
Journal of Cerebral Blood Flow & Metabolism (2015)