Abstract
Choice impulsivity has been linked to dopamine function and is consistently observed in attention deficit/hyperactivity disorder (ADHD) as a preference for smaller-immediate over larger-delayed rewards using choice-delay paradigms. More sophisticated delay discounting paradigms have yielded inconsistent results. Context and sample characteristics may have contributed to these variations. In this study we examine the effect of type (real vs hypothetical) and magnitude of reward as well as of variation in dopamine genes on choice impulsivity. We selected 36 male adolescents with ADHD-combined subtype (ADHD-CT) and 32 controls (mean age=15.42, SD=2.05) to form four roughly equally sized subgroups on the basis of DAT110/6 haplotype dosage (2 copies and <2 copies). Participants, who were also genotyped for the COMTval158met and DRD448bp−VNTR polymorphisms, performed a hypothetical and a real-time discounting task and provided self-ratings of trait impulsivity. The ADHD-CT group discounted rewards more steeply than controls only in the hypothetical task, with delay, but not reward magnitude, influencing choices. They also rated themselves as more impulsive compared with controls. DAT110/6 dosage and the COMTVal158Met genotype predicted trait impulsivity and discounting rates in the hypothetical task, but not in the real-time task. Our results directly link variation in genes putatively influencing dopamine signaling in the prefrontal cortex (COMTVal158Met) and the striatum (DAT110/6) with discounting rates in a hypothetical task (but not a real-time task) and self-ratings of trait impulsivity in ADHD-CT and healthy controls. The lack of magnitude effects in the hypothetical task suggests that discounting in this task may be influenced by different processes in ADHD-CT than in healthy controls.
Similar content being viewed by others
INTRODUCTION
Impulsive behavior is a key characteristic of several psychiatric conditions, including attention deficit/hyperactivity disorder (ADHD) (Moeller et al, 2001). One aspect of impulsivity is the preference for immediate gratification, even when waiting longer might lead to higher absolute gains (Evenden, 1999). The decrease in the subjective value of a reinforcer with time is called delay discounting and is observed in many species (Ainslie, 1975; Rachlin and Green, 1972). In humans, delay discounting is usually assessed through choices between hypothetical gains that systematically vary in magnitude or in the delay one needs to wait to receive them (Richards et al, 1999). The pattern of responses, determined by the rate at which an individual discounts potential gains as a function of time, is mathematically best described by a hyperbolic-like curve (Green and Myerson, 2004; Reynolds, 2006b). A steeper curve indicates a higher discounting rate, reflecting greater impulsivity or reduced self-control (Logue, 1988). Populations with impulse control problems consistently demonstrate higher discounting rates compared with healthy controls (see Green and Myerson, 2004; Reynolds, 2006b for reviews), and prospective studies suggest that high discounting rates may indicate vulnerability for the development of such problems across species (eg, substance abuse/dependence; Anker et al, 2009; Audrain-McGovern et al, 2009; Dandy and Gatch, 2009; Diergaarde et al, 2008; Perry et al, 2005; Perry et al, 2008; Poulos et al, 1995; Wilhelm and Mitchell, 2009).
Different lines of evidence have linked delay discounting to dopamine and the function of dopamine-modulated frontostriatal circuits (Adriani et al, 2009; Cardinal et al, 2001; Kable and Glimcher, 2009; Kobayashi and Schultz, 2008; Lee et al, 2009; Pothuizen et al, 2005). Dysfunctions in the neurobiological systems involved, from the cellular to the network level, are also postulated to give rise to changes in reinforcement sensitivity in ADHD (see, eg, Frank et al, 2007; Sagvolden et al, 2005; Tripp and Wickens, 2008; and see Luman et al, 2010 for a review). Pharmacological, genetic, and neuroimaging studies converge on the idea that ADHD is associated with deficiencies in dopamine signaling and abnormalities in dopamine-modulated frontostriatal circuits (Bush, 2010; Gizer et al, 2009; Krause et al, 2000; Paloyelis et al, 2007; Spencer et al, 2007; Volkow et al, 2009; Volkow et al, 2007).
As predicted by most of these neurobiological models (Luman et al, 2010), ADHD patients consistently prefer smaller-immediate over larger-delayed rewards using the choice-delay paradigm (Willcutt et al, 2008; see Paloyelis et al, 2009 for a review). In the choice-delay paradigm, participants make repeated choices between two standard amounts available at fixed delays. This paradigm is, however, faced with certain methodological issues: reduced sensitivity to detect individual differences in impulsivity, the confounding of time on task with choice preference, and concerns over the validity of using tertiary reinforcers to elicit impulsive behavior (Kuntsi et al, 2006b; Paloyelis et al, 2009).
Delay discounting paradigms overcome many of these problems, but have been used in few studies with ADHD patients, yielding somewhat inconsistent findings. In a study using a hypothetical discounting task, adolescents with ADHD and comorbid oppositional-defiant disorder made more impulsive choices than controls when the maximum hypothetical gain per trial was $100, but not when it was $1000 (Barkley et al, 2001). Using tasks presenting choices between real rewards and delays, Scheres et al (2006) did not find any differences, but in a later study where they examined ADHD subtypes separately they found that the ADHD-combined subtype (ADHD-CT) group, but not the inattentive subtype group, discounted rewards more steeply than controls (Scheres et al, 2010).
Experimentally measured discounting rates using hypothetical tasks show good temporal stability (Audrain-McGovern et al, 2009; Beck and Triplett, 2009; Kirby, 2009; Ohmura et al, 2006), similar to personality traits (Costa and McCrae, 1992), suggesting that they reflect a relatively stable individual difference. Real-time tasks, where participants experience the rewards and delays associated with each decision they make, are thought to be sensitive to state changes in impulsivity (McDonald et al, 2003; Reynolds et al, 2006; Reynolds and Schiffbauer, 2004). Evidence from acute pharmacological interventions with psychomotor stimulants and suppressants supports the greater sensitivity of real-time tasks to manipulations likely to induce changes in state impulsivity: discounting rates are affected when real-time task are used, but not in hypothetical tasks (Acheson and de Wit, 2008; Acheson et al, 2006; De Wit, 2009; McDonald et al, 2003; Richards et al, 1999). For example, methylphenidate reduced discounting rates in a real-time task but not in a hypothetical discounting task in ADHD patients (Shiels et al, 2009).
To our knowledge, no studies to date have examined the effects of variation in genes involved in dopamine signaling on impulsive decision making in adolescents, whereas a single study has examined the effects of COMTVal158Met in an adult sample of abstinent alcoholics and healthy participants (Boettiger et al, 2007). The present study was therefore designed to address these issues, focusing on the following three aims:
-
1)
To compare impulsive decision-making paradigms using a hypothetical and a real-time discounting task. We predicted that the ADHD-CT group would show higher discounting rates on both tasks and higher trait impulsivity scores on a self-report measure.
-
2)
To test if the impact of reward magnitude on the rate of discounting of hypothetical rewards differs depending on diagnosis. If delay exerts a greater influence in guiding impulsive choice in ADHD-CT compared with controls, as some theories would predict (Sonuga-Barke, 2005), we would expect that the effect of reward magnitude on discounting rates in the hypothetical task would be attenuated in the ADHD-CT group compared with controls (expecting a significant interaction between diagnosis and reward magnitude).
-
3)
To investigate whether common variants of the genes for the dopamine transporter (DAT110/6 haplotype) (Asherson et al, 2007; Brookes et al, 2006b) and the catechol-O-methyl transferase (COMTVal158Met) enzyme (Lachman et al, 1996), which are responsible for the bulk of dopamine degradation in the striatum and the prefrontal cortex, respectively (Akil et al, 2003; Chen et al, 2004; Lewis et al, 2001; Mazei et al, 2002; Morón et al, 2002), and a common variant of the dopamine D4-receptor gene (DRD448bp−VNTR) (Asghari et al, 1995) that has consistently been associated with ADHD (Brookes et al, 2006a; Gizer et al, 2009), can predict performance in measures of different aspects of impulsivity.
MATERIALS AND METHODS
Sample
In all, 68 boys of Caucasian origin (ADHD-CT=36; control=32) aged 11–20 years (M=15.42, SD=2.05) were recruited from a sample that had taken part in a previous study (Andreou et al, 2007; Kuntsi et al, in press; Wood et al, 2009) (see Table 1 for clinical and demographic data). The clinical group was part of the London branch of the International Multi-Centre ADHD Genetics (IMAGE) project (Chen et al, 2008; Kuntsi et al, 2006a; see Supplementary Material for details). Participants were selected based on DAT110/6 haplotype dosage (2 copies and <2 copies) (Asherson et al, 2007; Brookes et al, 2006b), forming four roughly equally sized subgroups. Participants receiving stimulant treatment for ADHD (72%) discontinued their medication for at least 48 h before testing (see Supplementary Material, Table S1 for details on medication history). Parents completed the long form of the revised Conners' Rating Scale (Conners et al, 1998) at the time of testing. In all, 30 participants (83%) had current total DSM-IV ADHD T-scores in the diagnostic range (with 67% scoring in the diagnostic range on both the inattention and hyperactivity/impulsivity subscales). Of the six participants with current total parent T-scores of <63 (the clinical cutoff in IMAGE; Marco et al, 2009), three were still receiving stimulant treatment. To include as many participants with ADHD as possible, we did not exclude participants with current parent ratings below the diagnostic range, but we repeated analyses excluding them. No comorbid disorder was associated with any of the three dopamine gene variants (see Supplementary Material, Tables S2–S4). Current levels of substance use were assessed using a self-report questionnaire (see Supplementary Material, Table S5). Analyses were repeated excluding four ADHD-CT participants with frequent or occasional use of drugs and/or heavy cigarette smoking (>5 cigarettes per day).
Control participants were aged matched to the clinical group. To exclude potential undiagnosed ADHD cases, participants with initial parent or teacher Conners' T-scores of >63 on any DSM-IV subscale were excluded. The DRD448bp−VNTR genotype could not be determined for one control participant, who was not included in some of the analyses.
Genotyping
Standard genotyping procedures were used (described in the Supplementary Material). DAT110/6 status (2 copies and <2 copies) was determined on the basis of zygosity for the constituent DAT1 polymorphisms (see Supplementary Material, Table S6). Frequencies for the DRD448bp−VNTR at exon 3 and the COMTVal158Met polymorphisms are presented in Supplementary Material (Table S7); no significant associations were observed with disease status. For statistical analyses we created binary groups for each genotype (COMTVal158Met: methionine homozygotes vs valine carriers; DRD448bp−VNTR: 7R-carriers vs rest).
Behavioral Measures of Impulsivity
Hypothetical delay discounting task
A computerized, adjusting-amount algorithm asked participants to make successive choices between a standard monetary amount available after a delay and a smaller adjusting amount available immediately (see Richards et al, 1999 for details on the adjusting-amount procedure). The subjective value at which a participant was indifferent between the larger-delayed and the smaller-immediate reward (indifference point) was estimated for eight delay intervals (1, 2, 7, 14, 30, 60, 120, and 180 days) using three standard amounts (£5, £15, and £30). Discounting curves were plotted for each participant and for each monetary magnitude using normalized indifference points (proportions of the standard amount) and delays (proportions of the maximum delay). Questions were presented in a random order. To increase motivation and accuracy, participants were told at the end of the task that the choice from one question, selected at random by themselves, would be honored. To keep the general level of compensation at acceptable levels for adolescents, we followed a credible procedure ensuring that the ‘randomly’ selected question always came from the £5 category.
Real-time delay discounting task
We used the UK version of the experiential discounting task (for a detailed description, see Supplementary Material) (Reynolds et al, 2006; Reynolds and Schiffbauer, 2004), in which participants experience the delays and receive the rewards associated with each decision they make. An indifference point was calculated for each of four choice blocks where participants made successive choices between a certain and immediate adjusting amount (initially £0.10) and a probabilistic (35%) £0.20 available at different delays (1, 7, 14, or 28 s depending on choice block). Because of the probabilistic nature of the larger amount (consistent across choice blocks), indifference points for each choice block were divided by the indifference point for the no-delay (1 s) choice block to control for individual differences in responses to probabilistic outcomes (Reynolds and Schiffbauer, 2004). The indifference points for the remaining blocks now reflected the relative change in discounting for each choice block compared with the no-delay block. This data adjustment and the systematic manipulation of delays render this task a measure of delay discounting, rather than risk discounting (Reynolds et al, 2006). Individual discounting curves were plotted after normalizing indifference points and delays (see description above). The four choice blocks were administered in random order after the participant had completed a practice session with the 7-s choice block.
Estimating discounting rates
In both tasks, discounting rates were assessed using the widely used (Scheres et al, 2010; Shiels et al, 2009) area-under-the-curve (AUC) method, which presents theoretical and practical advantages over alternatives (Beck and Triplett, 2009; Myerson et al, 2001). AUC values range from 0 to 1, with lower values indicating a higher rate of discounting and higher levels of impulsivity.
Self-Report Measures of Trait Impulsivity
Barratt's Impulsiveness Scale for Adolescents, version 11 (BIS-11A)
Adapted from the adult version (Patton et al, 1995; Stanford et al, 2009), it has been increasingly used with younger samples in many languages (Cosi et al, 2008; Fossati et al, 2002; Leshem and Glicksohn, 2007; von Diemen et al, 2007). With adult samples BIS-11 can differentiate between non-planning, attentional, and motor aspects of impulsivity (Patton et al, 1995; Stanford et al, 2009), yet a consistent factor structure is not reproduced with younger groups (Cosi et al, 2008; Fossati et al, 2002; Leshem and Glicksohn, 2007; von Diemen et al, 2007). Thus, the total scale score (ranging from 30 to 120) has been recommended (Fossati et al, 2002) and used (Melanko et al, 2009) as the most appropriate measure of trait impulsivity with adolescents. Higher scores reflect higher trait impulsivity levels.
Other Measures
ADHD rating scales
ADHD symptoms were assessed using the 18 DSM-IV items from the long form of the revised Conners' Parent Rating Scales (Conners et al, 1998).
General intelligence
The vocabulary, similarities, picture completion, and block design subtests of the Wechsler Intelligence scale for children III (Wechsler, 1991) or the Wechsler Intelligence Scale for Adults (Wechsler, 1997) were used to obtain an estimate of the child's IQ at the time of the initial assessment.
Socioeconomic status (SES)
The SES status of participants' families was estimated based on information about both parents' educational and occupational background using the Barratt Simplified Measure of Social Status (Barratt, 2006), which updated Hollingshead's Four-Factor Index of Social Status (Hollingshead, 1975) with a current occupation list (Baldwin and Dadds, 2007). Higher scores indicate higher socioeconomic levels.
Procedure
The discounting tasks (in counterbalanced order) and the BIS-11A questionnaire were administered under laboratory conditions in the afternoon session during a day-long visit to our research center as part of a larger study. Parent ratings were obtained during the same visit. Any cash earned during the tasks was exchanged for vouchers of equivalent value for the participant's preferred store. Ethics approval was obtained from the South London and Maudsley NHS Foundation Trust and informed consent was obtained from all participants.
Statistical Analyses
Age was covaried in all analyses; p-values after covarying IQ and SES are reported separately. A three-step approach was utilized to address the goals of this study.
First, separate MANCOVA/ANCOVA tests were conducted for each measure of impulsivity to test for an ADHD-CT deficit independently from genetic effects (obtaining results directly comparable to previous studies). The MANCOVA was used for the hypothetical discounting task, with the AUC measures for each reward magnitude used as the dependent variables and diagnosis (ADHC-CT vs control) as the between-subjects factor. Given the high correlations (Pearson's r from 0.80 to 0.94) in the discounting rates across magnitudes in the hypothetical task, we estimated a single overall AUC measure by averaging the three indifference points at each delay interval for each participant. This measure was used in an ANCOVA to estimate the effect size for the diagnosis effect and in subsequent genetic analyses.
Second, to test whether reward magnitude differentially affected the rate of discounting (AUC; reflecting the decrease in the subjective value of a reward with delay) of hypothetical rewards for the ADHD-CT and control groups, we used a mixed ANCOVA, with reward magnitude as the within-subjects factor (£5, £15, and £30) and diagnosis as the between-subjects factor.
Third, we tested for the main effects of DAT110/6, COMTval158met, and the DRD448bp−VNTR, and their interactions with diagnosis, using separate ANCOVAs for each measure of impulsivity. Family-wise error rate was contained at the α=0.05 level using a Bonferroni adjustment of the critical p-value for each ANCOVA to α=0.05/3=0.017. Similarly, a critical p-value of α=0.017/4=0.004 was used when simple effects analyses for significant or trend-level (<0.10) interactions were conducted. Owing to the relatively small sample and cell sizes, p-values obtained from parametric tests were confirmed using nonparametric procedures. We performed 10 000 random permutations on the age-regressed residuals for each measure and estimated p-values and 95% confidence intervals on the basis of Monte Carlo simulations.
All analyses were repeated without including the six ADHD participants with current T-scores of <63 on the parent Conners' total ADHD scale. In all cases, p-values and effect sizes either improved or remained approximately the same (see Supplementary Material). Similarly, excluding the four ADHD participants with frequent or occasional current use of cannabis (including two with frequent/occasional use of cocaine and all heavy smokers (>5 cigarettes per day)) did not change the observed pattern of results (see Supplementary Material). Pearson's correlation coefficients among ADHD ratings and the impulsivity measures are provided in Table 2.
RESULTS
Adolescents with ADHD-CT Displayed Higher Levels of Impulsivity
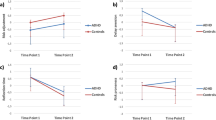
The ADHD-CT group displayed significantly steeper discounting rates (AUC) compared with controls in the hypothetical but not the real-time discounting task (see Table 3 and Figure 1). They also reported significantly higher levels of trait impulsivity. These effects remained significant after covarying IQ and SES.
Discounting curves for the hypothetical (overall discounting rate, group median) and real-time (group mean) delay discounting tasks for the attention deficit/hyperactivity disorder-combined subtype (ADHD-CT) and control groups. The graphs plot group indifference points—the subjective value (percentage of larger-delayed amount) at which participants were indifferent between the larger-delayed and the smaller-immediate reward—against delay intervals. In the real-time task, indifference points are adjusted for individual differences in response to probabilistic outcomes.
Delay but not Reward Magnitude Affected Discounting Rates (AUC) in the Hypothetical Discounting Task in the ADHD-CT Group
Discounting rates in the hypothetical task were submitted to a mixed ANCOVA with monetary magnitude (£5, £15, and £30) as the repeated factor and diagnosis as the between-subjects factor. The rate of discounting decreased as the magnitude of the monetary amount increased (magnitude effect: F(2, 132)=7.62, p=0.001; covarying IQ/SES: p<0.001), but only in the control group (magnitude × diagnosis: F(2, 132)=5.39, p=0.009; covarying IQ/SES: p=0.022; Figure 2; p-values were adjusted using the Greenhouse–Geisser epsilon).
Effect of monetary magnitude of the delayed amount on discounting rates (measured using the area under the curve) for adolescents with attention deficit/hyperactivity disorder-combined subtype (ADHD-CT) and control participants in the hypothetical delay discounting task.
DAT110/6 and COMTVal158Met Polymorphisms Predicted Discounting Rates (AUC) in the Hypothetical Task and Trait Impulsivity Ratings
Hypothetical discounting task
DAT110/6 status interacted with diagnosis (Table 4 and Figure 3a); this effect remained significant after covarying trait impulsivity scores (pcorrected<0.05; puncorrected=0.005; R2=0.086). Adolescents with ADHD-CT showed higher discounting rates than controls only among carriers of <2 copies of DAT110/6 (pcorrected<0.05; puncorrected<0.001); there was no group difference for DAT110/6 homozygotes (puncorrected=0.61). Furthermore, DAT110/6 homozygotes showed significantly higher discounting rates compared with those with <2 copies (pcorrected<0.05; puncorrected=0.002) among control participants. Numerically, the effect was in the opposite direction for the ADHD-CT group, but was not significant (puncorrected=0.12).
(a) Mean overall discounting rates (measured using the area under the curve (AUC)) in the hypothetical discounting task as a function of diagnosis (attention deficit/hyperactivity disorder-combined subtype (ADHD-CT) and controls) and DAT110/6 haplotype status (2 copies and <2 copies). Significance values from simple effects analyses are shown. (b) Self-reported scores on Barratt's Impulsiveness Scale (BIS-11A) as a function of diagnosis (ADHD-CT and controls) and DAT110/6 status (2 copies and <2 copies). Significance values from simple effects analyses are shown.
The COMTval158met polymorphism predicted discounting rates independent of diagnosis; covarying trait impulsivity ratings had no impact (pcorrected<0.05; puncorrected=0.013; R2=0.068). Met-allele homozygotes showed higher discounting rates than Val-allele carriers (pcorrected<0.05; puncorrected=0.014).
Real-time discounting task
No significant genetic effects were observed (Table 4).
Self-reported trait impulsivity ratings
DAT110/6 status predicted trait impulsivity ratings independent of diagnosis (Table 4 and Figure 3b). DAT110/6 homozygotes reported lower impulsivity levels than carriers of <2 copies, an effect that remained highly significant after covarying discounting rates in the hypothetical task (pcorrected<0.05; puncorrected=0.004; R2=0.095). A trend for a diagnosis × DAT110/6 (puncorrected<0.10) suggested that this effect might be significant only in the ADHD-CT group (pcorrected<0.05; puncorrected=0.002; control group: puncorrected>0.10). It further indicated that adolescents with ADHD-CT reported higher levels of impulsivity compared with controls if they carried <2 DAT110/6 copies (pcorrected<0.05; puncorrected<0.001) but not among DAT110/6 homozygotes (puncorrected=0.13).
There were no effects for the DRD448bp−VNTR polymorphism on any on the impulsivity measures (see Table 4).
DISCUSSION
This study used a relatively homogeneous sample of male adolescents with ADHD-CT and typically developing controls, and reports three novel findings. First, when comparing performance on a hypothetical and a real-time discounting task, we found that the ADHD-CT group showed steeper discounting rates (smaller AUC, denoting higher levels of impulsivity) in the hypothetical task, but not when rewards/delays were real. The ADHD-CT group also reported higher trait impulsivity self-ratings than controls. Second, discounting rates in the hypothetical task were influenced by delay but not reward magnitude for the ADHD-CT group. Third, DAT110/6 haplotype dosage and the COMTVal158Met genotype predicted discounting rates in the hypothetical discounting task (but not the real-time task) and trait impulsivity ratings.
The contrast between discounting rates in the hypothetical and real-time tasks in terms of diagnostic group and gene effects is novel. It cannot be attributed to validity issues with the real-time task, as both groups showed discounting in this study, supporting evidence from earlier studies (Fields et al, 2009; Krishnan-Sarin et al, 2007; Melanko et al, 2009). These data therefore suggest that discounting rates in hypothetical and real-time tasks may reflect different aspects of impulsivity. As discussed in the Introduction, one possible distinction is that performance in the hypothetical task putatively reflects a relatively stable individual difference (an aspect of trait impulsivity), whereas performance in the real-time task may be more sensitive to the transient effects of factors affecting state impulsivity, such as acute pharmacological interventions (Shiels et al, 2009) or task characteristics. Further support for this distinction is provided by the lack of significant correlations between discounting rates in the real-time task and trait impulsivity scores, or discounting rates in the hypothetical task, which is consistent with the literature (Fields et al, 2009; Krishnan-Sarin et al, 2007; Melanko et al, 2009; Reynolds et al, 2008; Reynolds et al, 2006) with few exceptions (Meda et al, 2009; Reynolds, 2006a).
Although impulsive decision making in discounting paradigms is consistent with the pattern of neurobiological deficits observed in ADHD and predicted by most neurobiological models of the disorder (see Luman et al, 2010 for a review), such models do not distinguish between performance in real-time and hypothetical paradigms, as most of them imply context-invariant deficits. One possible explanation for the lack of case–control differences in the real-time discounting task could be that decision making in this task (where participants experience the delays and rewards associated with each choice they make) impacts on an individual's current state of arousal considerably more than in the hypothetical task, which consequently might induce changes in state impulsivity (to which real-time tasks are assumed to be more sensitive). As shown using reaction time performance paradigms, the performance of ADHD patients tends to approach or equal control levels under task conditions that increase stimulation/arousal levels (Andreou et al, 2007; Johnson et al, 2007a; Johnson et al, 2007b; Konrad et al, 2000; Kuntsi et al, 2009; McInerney and Kerns, 2003; O'Connell et al, 2008; Slusarek et al, 2001; Uebel et al, 2010). Therefore, it is possible that discrepancies among studies using real-time tasks (eg, this study and Scheres et al, 2010) might be explained by altered arousal-regulation processes in ADHD. This could occur if case–control differences in measures of state impulsivity depend on the extent to which specific task characteristics alter arousal levels, because of differences in factors such as the monotony of the task, salience of the reward, and length and expectancy of the delay. This explanatory framework leads to specific predictions that can be investigated by examining the relationship between indices of arousal-regulation deficits and performance in a range of real-time discounting paradigms.
Another novel finding in this study was the lack of reward magnitude effects in the hypothetical task for the ADHD-CT group, suggesting that their choices were influenced only by delay. Discounting rates in the control group were inversely related to reward magnitude, consistent with the literature (Chapman and Winquist, 1998; Kirby and Marakovi, 1996; Myerson and Green, 1995; Smith and Hantula, 2008). This discrepancy indicates that different processes might drive discounting in ADHD-CT and controls. One possible explanation could be that ADHD-CT participants were particularly sensitive to delays, as would be predicted by delay aversion theory (Sonuga-Barke, 2005), thus overriding the effect of reward magnitude, at least when choices do not affect current state and with the range of values used in this study.
The predictive value of variants for the DAT1 and COMT genes was restricted to measures of relatively stable aspects of impulsivity. Previous studies have linked the DAT1 9-repeat allele (one of the two polymorphisms constituting the haplotype studied here) with increased ventral striatal reactivity during reward anticipation (Forbes et al, 2009), which in turn had been linked with steeper hypothetical discounting rates (Hariri et al, 2006). In this study we extend this work, showing that DAT110/6 dosage directly predicted hypothetical discounting rates and trait impulsivity in ADHD-CT and controls. Previous studies had failed to show a reliable effect of DAT1 genotype on executive functions/response inhibition (Rommelse et al, 2008), although a study focusing on the same DAT110/6 haplotype (Bellgrove et al, 2009) reported a significant interaction of diagnosis with spatial attention measures explaining 4.9–5.8% of the variance. In our study, DAT110/6 interacted with diagnosis in the hypothetical task, explaining 11.7% of the variance. Although the DAT110/6 interaction with diagnosis was only a trend for trait impulsivity ratings, it accounted for 4.7% of the variance, which is comparable to the effect sizes observed with the same haplotype in the study by Bellgrove et al (2009).
Post hoc analyses of the interaction effect (controlling for multiple testing) revealed remarkably similar effects of DAT110/6 dosage across measures, providing a within-sample replication of the effect. The differential effects of DAT110/6 dosage depending on diagnostic status (and the reverse) are not entirely surprising. Dopamine effects on neurocognitive functions have been shown to reflect an inverted-U shape (Goldman-Rakic et al, 2000). Therefore, assuming that there is some optimal level of dopamine modulating the function of frontal-striatal circuits underlying impulsive behavior, any factor disturbing this balance in either direction would be likely to impair behavior (Egan et al, 2001; Goldberg et al, 2003; Williams-Gray et al, 2007).
Steeper discounting rates in the hypothetical task were also associated with homozygosity for the COMTVal158Met Met-allele (which substantially reduces dopamine catabolism in the prefrontal cortex) (Chen et al, 2004; Lachman et al, 1996; Lotta et al, 1995), independent of diagnosis (R2=6.9%). This finding is consistent with evidence showing that the dopamine-modulated prefrontal cortex has a key role in choice impulsivity (Kable and Glimcher, 2007; Kable and Glimcher, 2009; Kheramin et al, 2004; McClure et al, 2007; McClure et al, 2004; Winstanley et al, 2006). The role of COMTVal158Met in impulsivity has received scant attention to date, with the Met-allele being associated with reduced immediate-reward bias (Boettiger et al, 2007) and increased novelty seeking (Golimbet et al, 2007). Variants of COMTVal158Met and MAOA (which perform most of the catecholamine catabolism in the prefrontal cortex) (Chen et al, 2004; Gogos et al, 1998; Huotari et al, 2002; Matsumoto et al, 2003; Tunbridge et al, 2004; Volavka et al, 2004) with reduced enzymatic activity have been associated with aggressive impulsivity and suicide (Contini et al, 2006; Huang et al, 2004; Volavka et al, 2004). Meta-analytic evidence has not associated ADHD with COMTVal158Met (Gizer et al, 2009), although some evidence suggests that gender could be a moderating factor with Met being the risk allele in boys (Biederman et al, 2008; Qian et al, 2003). This is consistent with evidence that COMTVal158Met is differentially expressed across genders (Dempster et al, 2006; Gogos et al, 1998). A recent study confirmed the association of the Met-allele with increased risk for ADHD in children in a predominantly male (84%) sample, and also reported that Met was associated with increased symptom severity (Pálmason et al, 2010). Moreover, Met has been associated with ADHD inattention and hyperactivity impulsivity symptoms in adult community samples (Ettinger et al, 2006; Gothelf et al, 2007; Michaelovsky et al, 2008; Reuter et al, 2006). The DRD448bp−VNTR was not associated with any impulsivity measure, in line with a recent meta-analytic review (which suggested that another marker on the gene might be related to impulsivity; Munafò et al, 2008).
Although our data confirmed our hypotheses that variation in DAT110/6 and COMTVal158Met predicts behavioral impulsivity, the precise mechanisms mediating these effects, in terms of dopamine signaling or the neural processes involved, remain unknown. One reason is the lack of sufficient evidence regarding the precise functional effects of these genetic variants. For example, although the DAT13′UTR 48 base-pair VNTR polymorphism (part of the DAT110/6 haplotype) has been associated with alterations in gene expression, evidence regarding the impact of specific variants is inconsistent (for a review, see van de Giessen et al, 2009). Second, even when the effects of genetic variation in terms of gene expression and dopamine catabolism are better understood (eg, COMTVal158Met), predicting the precise role of a variant on behavioral impulsivity may still be elusive, given that it is likely to depend on the dynamic interaction among many factors and reflect the outcome of long-term adaptation processes. For example, the DAT1 10-repeat allele is considered to increase risk for ADHD in children and adolescents, yet in adults (and thus, by definition, in persisting forms of the disorder) ADHD has been associated with the 9-repeat allele (Franke et al, 2008; Franke et al, 2010). Our study, alongside recent work (Bellgrove et al, 2009), has documented that variation in DAT110/6 modulates behavioral outcomes, highlighting the need for a better understanding of the impact of this haplotype on gene expression, dopamine signaling, and neural mechanisms involved. Some promising initial evidence has indicated that the DAT13′UTR polymorphism may be modulating the responsivity of the striatum during a response inhibition and a reward-processing paradigm (Durston et al, 2008; Forbes et al, 2009).
Our genetic findings finally suggest that the rate of experimental discounting and trait impulsivity ratings reflect distinct aspects of impulsivity. Covarying trait impulsivity ratings or discounting rates when examining the genetic effects on either measure left effect sizes virtually unchanged and results remained significant. This finding provides no support for the mediation of gene effects on discounting by impulsive behavior (or the reverse), but rather that the two measures of relatively stable impulsivity predispositions (hypothetical discounting and self-report rating scale) represent pleiotropic effects (multiple outcomes) of the genetic influences.
In summary, the pattern of findings in this study distinguishes between aspects of impulsivity by demonstrating specific effects of diagnosis and variation in dopamine genes (DAT110/6 and COMTVal158Met) on behavioral and laboratory measures assumed to reflect relatively stable aspects of impulsivity (discounting in the hypothetical task and trait impulsivity self-ratings) but not on discounting rates in a real-time task, which may be more sensitive to factors impacting on state impulsivity levels. However, our study was not designed to distinguish between trait and state aspects of impulsivity, or test the various models predicting impulsive behavior in ADHD; these goals should be addressed in future research. Our data suggest that existing ADHD models need to distinguish between trait and state aspects of impulsivity, and that arousal regulation should be further investigated as one potential mechanism contributing to real-time impulsive decision making in ADHD. Future studies should also employ a wider range of real-time paradigms. The sample size is a limitation of this study; the genetic findings should be considered as preliminary until confirmed within a larger sample, which should also examine gene-by-gene interactions and assess the impact of potential confounds pertaining to the ADHD-CT group, such as possible gene interactions with the effects of long-term stimulant treatment.
References
Acheson A, de Wit H (2008). Bupropion improves attention but does not affect impulsive behavior in healthy young adults. Exp Clin Psychopharmacol 16: 113–123.
Acheson A, Reynolds B, Richards JB, De Wit H (2006). Diazepam impairs behavioral inhibition but not delay discounting or risk taking in healthy adults. Exp Clin Psychopharmacol 14: 190–193.
Adriani W, Boyer F, Gioiosa L, Macri S, Dreyer JL, Laviola G (2009). Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats' nucleus accumbens. Neuroscience 159: 47–58.
Ainslie G (1975). Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull 82: 463–496.
Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE (2003). Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci 23: 2008–2013.
Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A et al (2007). Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychol Med 37: 1703–1715.
Anker JJ, Perry JL, Gliddon LA, Carroll ME (2009). Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav 93: 343–348.
Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH (1995). Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem 65: 1157–1165.
Asherson P, Brookes K, Franke B, Chen W, Gill M, Ebstein RP et al (2007). Confirmation that a specific haplotype of the dopamine transporter gene is associated with combined-type ADHD. Am J Psychiatry 164: 674–677.
Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP (2009). Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend 103: 99–106.
Baldwin JS, Dadds MR (2007). Reliability and validity of parent and child versions of the multidimensional anxiety scale for children in community samples. J Am Acad Child Adolesc Psychiatry 46: 252–260.
Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L (2001). Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD). J Abnorm Child Psychol 29: 541–556.
Barratt W (2006). The Barratt Simplified Measure of Social Status. (Copy available online at: http://wbarratt.indstate.edu/socialclass/Barratt_Simplifed_Measure_of_Social_Status.pdf ).
Beck RC, Triplett MF (2009). Test-retest reliability of a group-administered paper-pencil measure of delay discounting. Exp Clin Psychopharmacol 17: 345–355.
Bellgrove M, Johnson K, Barry E, Mulligan A, Hawi Z, Gill M et al (2009). Dopaminergic haplotype as a predictor of spatial inattention in children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 66: 1135–1142.
Biederman J, Kim JW, Doyle AE, Mick E, Fagerness J, Smoller JW et al (2008). Sexually dimorphic effects of four genes (COMT, SLC6A2, MAOA, SLC6A4) in genetic associations of ADHD: a preliminary study. Am J Med Genet B Neuropsychiatr Genet 147B: 1511–1518.
Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D′Esposito M et al (2007). Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci 27: 14383–14391.
Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N et al (2006a). The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry 11: 934–953.
Brookes KJ, Mill J, Guindalini C, Curran S, Xu X, Knight J et al (2006b). A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. Arch Gen Psychiatry 63: 74–81.
Bush G (2010). Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology 35: 278–300.
Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ (2001). Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292: 2499–2501.
Chapman GB, Winquist JR (1998). The magnitude effect: temporal discount rates and restaurant tips. Psychon B Rev 5: 119–123.
Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S et al (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75: 807–821.
Chen W, Zhou K, Sham P, Franke B, Kuntsi J, Campbell D et al (2008). DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. Am J Med Genet B Neuropsychiatr Genet 147B: 1450–1460.
Conners CK, Sitarenios G, Parker JD, Epstein JN (1998). The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 26: 257–268.
Contini V, Marques FZC, Garcia CED, Hutz MH, Bau CHD (2006). MAOA-uVNTR polymorphism in a Brazilian sample: further support for the association with impulsive behaviors and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet 141B: 305–308.
Cosi S, Vigil-Colet A, Canals J, Lorenzo-Seva U (2008). Psychometric properties of the Spanish adaptation of the Barratt Impulsiveness Scale-11-A for children. Psychol Rep 103: 336–346.
Costa PT, McCrae RR (1992). Revised NEO Personality Inventory (NEO–PI–R) and NEO Five-Factor Inventory (NEO–FFI) Professional Manual. Psychological Assessment Resources: Odessa, FL.
Dandy KL, Gatch MB (2009). The effects of chronic cocaine exposure on impulsivity in rats. Behav Pharmacol 20: 400–405.
De Wit H (2009). Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14: 22–31.
Dempster EL, Mill J, Craig IW, Collier DA (2006). The quantification of COMT mRNA in post mortem cerebellum tissue: diagnosis, genotype, methylation and expression. BMC Med Genet 7: 10.
Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN et al (2008). Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63: 301–308.
Durston S, Fossella JA, Mulder MJ, Casey BJ, Ziermans TB, Vessaz MN et al (2008). Dopamine transporter genotype conveys familial risk of attention-deficit/hyperactivity disorder through striatal activation. J Am Acad Child Adolesc Psychiatry 47: 61–67.
Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE et al (2001). Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 98: 6917–6922.
Ettinger U, Joober R, DE Guzman R, O'driscoll GA (2006). Schizotypy, attention deficit hyperactivity disorder, and dopamine genes. Psychiatry Clin Neurosci 60: 764–767.
Evenden JL (1999). Varieties of impulsivity. Psychopharmacology (Berl) 146: 348–361.
Fields S, Collins C, Leraas K, Reynolds B (2009). Dimensions of impulsive behavior in adolescent smokers and nonsmokers. Exp Clin Psychopharmacol 17: 302–311.
Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR (2009). Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry 14: 60–70.
Fossati A, Barratt ES, Acquarini E, Di Ceglie A (2002). Psychometric properties of an adolescent version of the Barratt Impulsiveness Scale-11 for a sample of Italian high school students. Percept Mot Skills 95: 621–635.
Frank MJ, Santamaria A, O′Reilly RC, Willcutt E (2007). Testing computational models of dopamine and noradrenaline dysfunction in attention deficit/hyperactivity disorder. Neuropsychopharmacology 32: 1583–1599.
Franke B, Hoogman M, Arias Vasquez A, Heister JGaM, Savelkoul PJ, Naber M et al (2008). Association of the dopamine transporter (SLC6A3/DAT1) gene 9-6 haplotype with adult ADHD. Am J Med Genet B Neuropsychiatr Genet 147B: 1576–1579.
Franke B, Vasquez AA, Johansson S, Hoogman M, Romanos J, Boreatti-Hümmer A et al (2010). Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology 35: 656–664.
Gizer IR, Ficks C, Waldman ID (2009). Candidate gene studies of ADHD: a meta-analytic review. Hum Genet 126: 51–90.
Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D et al (1998). Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 95: 9991–9996.
Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS et al (2003). Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 60: 889–896.
Goldman-Rakic PS, Muly III EC, Williams GV (2000). D1 receptors in prefrontal cells and circuits. Brain Res Rev 31: 295–301.
Golimbet VE, Alfimova MV, Gritsenko IK, Ebstein RP (2007). Relationship between dopamine system genes and extraversion and novelty seeking. Neurosci Behav Physiol 37: 601–606.
Gothelf D, Michaelovsky E, Frisch A, Zohar AH, Presburger G, Burg M et al (2007). Association of the low-activity COMT 158Met allele with ADHD and OCD in subjects with velocardiofacial syndrome. Int J Neuropsychopharmacol 10: 301–308.
Green L, Myerson J (2004). A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull 130: 769–792.
Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB (2006). Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci 26: 13213–13217.
Hollingshead AA (1975). Four-Factor Index of Social Status. Yale University: New Haven, CT.
Huang YY, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ (2004). An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology 29: 1498–1505.
Huotari M, Gogos JA, Karayiorgou M, Koponen O, Forsberg M, Raasmaja A et al (2002). Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur J Neurosci 15: 246–256.
Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M et al (2007a). Response variability in attention deficit hyperactivity disorder: evidence for neuropsychological heterogeneity. Neuropsychologia 45: 630–638.
Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Daibhis A et al (2007b). Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia 45: 2234–2245.
Kable JW, Glimcher PW (2007). The neural correlates of subjective value during intertemporal choice. Nat Neurosci 10: 1625–1633.
Kable JW, Glimcher PW (2009). The neurobiology of decision: consensus and controversy. Neuron 63: 733–745.
Kheramin S, Body S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E et al (2004). Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology (Berl) 175: 206–214.
Kirby KN (2009). One-year temporal stability of delay-discount rates. Psychon Bull Rev 16: 457–462.
Kirby KN, Marakovi NN (1996). Delay-discounting probabilistic rewards: rates decrease as amounts increase. Psycho Bull Rev 3: 100–104.
Kobayashi S, Schultz W (2008). Influence of reward delays on responses of dopamine neurons. J Neurosci 28: 7837–7846.
Konrad K, Gauggel S, Manz A, Scholl M (2000). Lack of inhibition: a motivational deficit in children with attention deficit/hyperactivity disorder and children with traumatic brain injury. Child Neuropsychol 6: 286–296.
Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K (2000). Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett 285: 107–110.
Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A et al (2007). Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend 88: 79–82.
Kuntsi J, Neale BM, Chen W, Faraone SV, Asherson P (2006a). The IMAGE project: methodological issues for the molecular genetic analysis of ADHD. Behav Brain Funct 2: 27.
Kuntsi J, Rogers H, Swinard G, Borger N, van der Meere J, Rijsdijk F et al (2006b). Reaction time, inhibition, working memory and ‘delay aversion’ performance: genetic influences and their interpretation. Psychol Med 36: 1613–1624.
Kuntsi J, Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B et al (in press). Separation of cognitive impairments in attention deficit hyperactivity disorder into two familial factors. Arch Gen Psychiatry.
Kuntsi J, Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B (2009). Why cognitive performance in ADHD may not reveal true potential: findings from a large population-based sample. J Int Neuropsychol Soc 15: 570–579.
Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM (1996). Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6: 243–250.
Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR et al (2009). Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29: 14734–14740.
Leshem R, Glicksohn J (2007). The construct of impulsivity revisited. Pers Indiv Differ 43: 681–691.
Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A (2001). Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol 432: 119–136.
Logue AW (1988). Research on self-control: an integrating framework. Behav Brain Sci 11: 665–709.
Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I et al (1995). Kinetics of human soluble and membrane-bound catechol 0-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry (Mosc) 34: 4202–4210.
Luman M, Tripp G, Scheres A (2010). Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci Biobehav Rev 34: 744–754.
Marco R, Miranda A, Schlotz W, Melia A, Mulligan A, Muller U et al (2009). Delay and reward choice in ADHD: an experimental test of the role of delay aversion. Neuropsychology 23: 367–380.
Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM et al (2003). Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology 28: 1521–1530.
Mazei MS, Pluto CP, Kirkbride B, Pehek EA (2002). Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res 936: 58–67.
McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD (2007). Time discounting for primary rewards. J Neurosci 27: 5796–5804.
McClure SM, Laibson DI, Loewenstein G, Cohen JD (2004). Separate neural systems value immediate and delayed monetary rewards. Science 306: 503–507.
McDonald J, Schleifer L, Richards JB, de Wit H (2003). Effects of THC on behavioral measures of impulsivity in humans. 28: 1356–1365.
McInerney RJ, Kerns KA (2003). Time reproduction in children with ADHD: motivation matters. Child Neuropsychol 9: 91–108.
Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM et al (2009). Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behav Pharmacol 20: 390–399.
Melanko S, Leraas K, Collins C, Fields S, Reynolds B (2009). Characteristics of psychopathy in adolescent nonsmokers and smokers: relations to delay discounting and self reported impulsivity. Exp Clin Psychopharmacol 17: 258–265.
Michaelovsky E, Gothelf D, Korostishevsky M, Frisch A, Burg M, Carmel M et al (2008). Association between a common haplotype in the COMT gene region and psychiatric disorders in individuals with 22q11.2DS. Int J Neuropsychopharmacol 11: 351–363.
Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC (2001). Psychiatric aspects of impulsivity. Am J Psychiatry 158: 1783–1793.
Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002). Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22: 389–395.
Munafò MR, Yalcin B, Willis-Owen SA, Flint J (2008). Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: meta-analysis and new data. Biol Psychiatry 63: 197–206.
Myerson J, Green L (1995). Discounting of delayed rewards—models of individual choice. J Exp Anal Behav 64: 263–276.
Myerson J, Green L, Warusawitharana M (2001). Area under the curve as a measure of discounting. J Exp Anal Behav 76: 235–243.
O'Connell RG, Bellgrove MA, Dockree PM, Lau A, Fitzgerald M, Robertson IH (2008). Self-alert training: volitional modulation of autonomic arousal improves sustained attention. Neuropsychologia 46: 1379–1390.
Ohmura Y, Takahashi T, Kitamura N, Wehr P (2006). Three-month stability of delay and probability discounting measures. Exp Clin Psychopharmacol 14: 318–328.
Pálmason H, Moser D, Sigmund J, Vogler C, Hänig S, Schneider A et al (2010). Attention-deficit/hyperactivity disorder phenotype is influenced by a functional catechol-O-methyltransferase variant. J Neural Transm 117: 259–267.
Paloyelis Y, Asherson P, Kuntsi J (2009). Are ADHD symptoms associated with delay aversion or choice impulsivity? A general population study. J Am Acad Child Adolesc Psychiatry 48: 837–846.
Paloyelis Y, Mehta MA, Kuntsi J, Asherson P (2007). Functional MRI in ADHD: a systematic literature review. Expert Rev Neurother 7: 1337–1356.
Patton JH, Stanford MS, Barratt ES (1995). Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51: 768–774.
Perry JL, Larson EB, German JP, Madden GJ, Carroll ME (2005). Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 178: 193–201.
Perry JL, Nelson SE, Carroll ME (2008). Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol 16: 165–177.
Pothuizen HH, Jongen-Relo AL, Feldon J, Yee BK (2005). Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur J Neurosci 22: 2605–2616.
Poulos CX, Le AD, Parker JL (1995). Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol 6: 810–814.
Qian Q, Wang Y, Zhou R, Li J, Wang B, Glatt S et al (2003). Family-based and case-control association studies of catechol-O-methyltransferase in attention deficit hyperactivity disorder suggest genetic sexual dimorphism. Am J Med Genet B Neuropsychiatr Genet 118B: 103–109.
Rachlin H, Green L (1972). Commitment, choice and self-control. J Exp Anal Behav 17: 15–22.
Reuter M, Kirsch P, Hennig J (2006). Inferring candidate genes for attention deficit hyperactivity disorder (ADHD) assessed by the World Health Organization Adult ADHD Self-Report Scale (ASRS). J Neural Transm (Vienna) 113: 929–938.
Reynolds B (2006a). The experiential discounting task is sensitive to cigarette-smoking status and correlates with a measure of delay discounting. Behav Pharmacol 17: 133–142.
Reynolds B (2006b). A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol 17: 651–667.
Reynolds B, Penfold RB, Patak M (2008). Dimensions of impulsive behavior in adolescents: laboratory behavioral assessments. Exp Clin Psychopharmacol 16: 124–131.
Reynolds B, Richards JB, de Wit H (2006). Acute-alcohol effects on the experiential discounting task (EDT) and a question-based measure of delay discounting. Pharmacol Biochem Behav 83: 194–202.
Reynolds B, Schiffbauer R (2004). Measuring state changes in human delay discounting: an experiential discounting task. Behav Processes 67: 343–356.
Richards JB, Zhang L, Mitchell SH, de Wit H (1999). Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav 71: 121–143.
Rommelse NNJ, Altink ME, Arias-Vásquez A, Buschgens CJM, Fliers E, Faraone SV et al (2008). A review and analysis of the relationship between neuropsychological measures and DAT1 in ADHD. Am J Med Genet B Neuropsychiatr Genet 147B: 1536–1546.
Sagvolden T, Johansen EB, Aase H, Russell VA (2005). A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci 28: 397–419.
Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E et al (2006). Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia 44: 2092–2103.
Scheres A, Tontsch C, Thoeny AL, Kaczkurkin A (2010). Temporal reward discounting in attention-deficit/hyperactivity disorder: the contribution of symptom domains, reward magnitude, and session length. Biol Psychiatry 67: 641–648.
Shiels K, Hawk LW, Reynolds B, Mazzullo RJ, Rhodes JD, Pelham WE et al (2009). Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol 17: 291–301.
Slusarek M, Velling S, Bunk D, Eggers C (2001). Motivational effects on inhibitory control in children with ADHD. J Am Acad Child Adolesc Psychiatry 40: 355–363.
Smith CL, Hantula DA (2008). Methodological considerations in the study of delay discounting in intertemporal choice: a comparison of tasks and modes. Behav Res Methods 40: 940–953.
Sonuga-Barke EJS (2005). Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry 57: 1231–1238.
Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E et al (2007). Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry 62: 1059–1061.
Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH (2009). Fifty years of the Barratt Impulsiveness Scale: an update and review. Pers Indiv Differ 47: 385–395.
Tripp G, Wickens JR (2008). Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. J Child Psychol Psychiatry 49: 691–704.
Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ (2004). Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci 24: 5331–5335.
Uebel H, Albrecht B, Asherson P, Borger NA, Butler L, Chen W et al (2010). Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. J Child Psychol Psychiatry 51: 210–218.
van de Giessen EM, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J (2009). Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med 50: 45–52.
Volavka J, Bilder R, Nolan K (2004). Catecholamines and aggression: the role of COMT and MAO polymorphisms. Ann NY Acad Sci 1036: 393–398.
Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F et al (2009). Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA 302: 1084–1091.
Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS et al (2007). Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 64: 932–940.
von Diemen L, Szobot CM, Kessler F, Pechansky F (2007). Adaptation and construct validation of the Barratt Impulsiveness Scale (BIS 11) to Brazilian Portuguese for use in adolescents. Rev Bras Psiquiatr 29: 153–156.
Wechsler D (1991). Wechsler Intelligence Scale for Children, 3rd edn. The Psychological Corporation: London.
Wechsler D (1997). Wechsler Intelligence Scale for Adults. The Psychological Corporation: London.
Wilhelm CJ, Mitchell SH (2009). Strain differences in delay discounting using inbred rats. Genes Brain Behav 8: 426–434.
Willcutt E, Sonuga-Barke E, Nigg J, Sergeant J (2008). Recent developments in neuropsychological models of childhood psychiatric disorders. In: Banaschewski T, Rhode LA (eds). Biological Child Psychiatry. Recent Trends and Developments, vol. 24. Karger: Basel. pp 195–226.
Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA (2007). Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci 27: 4832–4838.
Winstanley CA, Theobald DEH, Dalley JW, Cardinal RN, Robbins TW (2006). Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex 16: 106–114.
Wood AC, Asherson P, Rijsdijk F, Kuntsi J (2009). Is overactivity a core feature in ADHD? Familial and receiver operating characteristic curve analysis of mechanically assessed activity level. J Am Acad Child Adolesc Psychiatry 10: 1023–1030.
Acknowledgements
This work was supported in part by UK Medical Research Council Grant G03001896 to J Kuntsi and NIH grants R01MH62873 and R01MH081803 to SV Faraone. We thank all who make this research possible: our participants and their families, who give their time and support so unstintingly, and our research team, Kelly Harris and Chloe Booth. We also thank Dr Brady Reynolds for his permission to use the Experiential Discounting Task, and his advice on analyzing the data obtained from this task.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
PA has given sponsored talks and/or been an advisor for Shire, Janssen-Cilag, Eli-Lilly, Flynn Pharma, and Pfizer, regarding the diagnosis and treatment of ADHD; all funds have been donated to the University Research Fund for studies of ADHD. MAM receives research funding from Eli Lilly and is a scientific advisor to Cambridge Cognition. SVF has, in the past year, received consulting fees and has been on Advisory Boards for Eli Lilly, Ortho-McNeil, and Shire Development and has received research support from Eli Lilly, Pfizer, Shire, and the National Institutes of Health. In previous years, SVF has received consulting fees or has been on Advisory Boards or has been a speaker for the following sources: Shire, McNeil, Janssen, Novartis, Pfizer, and Eli Lilly. In previous years, he has received research support from Eli Lilly, Shire, Pfizer, and the National Institutes of Health. JK has received a speaker's fee from Eli Lilly that has been used for educational and research activities.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Paloyelis, Y., Asherson, P., Mehta, M. et al. DAT1 and COMT Effects on Delay Discounting and Trait Impulsivity in Male Adolescents with Attention Deficit/Hyperactivity Disorder and Healthy Controls. Neuropsychopharmacol 35, 2414–2426 (2010). https://doi.org/10.1038/npp.2010.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2010.124
Keywords
This article is cited by
-
The Ghrelin Antagonist [D-Lys3]-GHRP-6 Decreases Signs of Risk Behavior in a Model of Gambling Addiction in Rats by Altering Dopamine and Serotonin Metabolism
Neuroscience and Behavioral Physiology (2022)
-
Attention-Deficit/Hyperactivity Disorder Symptoms Are Associated with Greater Delay Discounting of Condom-Protected Sex and Money
Archives of Sexual Behavior (2021)
-
Intrinsic non-hub connectivity predicts human inter-temporal decision-making
Brain Imaging and Behavior (2021)
-
Dissociable fronto-striatal functional networks predict choice impulsivity
Brain Structure and Function (2020)
-
Linking Delay Discounting and Substance Use Disorders: Genotypes and Phenotypes
Perspectives on Behavior Science (2019)