Abstract
Catecholamines, particularly dopamine, have been implicated in various aspects of the reward function including the ability to learn through reinforcement and to modify flexibly responses to changing reinforcement contingencies. We examined the impact of catecholamine depletion (CD) achieved by oral administration of alpha-methyl-paratyrosine (AMPT) on probabilistic reversal learning and passive avoidance (PA) in 15 female subjects with major depressive disorder in full remission (RMDD) and 12 healthy female controls. The CD did not affect significantly the acquisition phase of the reversal learning task. However, CD selectively impaired reversal of the 80–20 contingency pair. In the PA learning task, CD was associated with reduced responding toward rewarding stimuli, although the RMDD and control subjects did not differ regarding these CD-induced changes in reward processing. Interestingly, the performance decrement produced by AMPT on both of these tasks was associated with the level of decreased metabolism in the perigenual anterior cingulate cortex. In an additional examination using the affective Stroop task we found evidence for impaired executive attention as a trait abnormality in MDD. In conclusion, this study showed specific effects of CD on the processing of reward-related stimuli in humans and confirms earlier investigations that show impairments of executive attention as a neuropsychological trait in affective illness.
Similar content being viewed by others
INTRODUCTION
Abnormalities of catecholaminergic neurotransmitter systems have been implicated in various neuropsychiatric conditions including depression and addiction (Volkow et al, 2004; Dunlop and Nemeroff, 2007; Hasler et al, 2008). Although catecholaminergic neurotransmission is thought to be reduced in these disorders, the specific contributions of catecholamines to attention, cognition, and affect remain unclear. An instructive paradigm for investigating the relationship between catecholaminergic function and behavior has involved the behavioral response to catecholamine depletion (CD), achieved by oral administration of alpha-methyl-paratyrosine (AMPT) (Berman et al, 1999; Hasler et al, 2004). AMPT is a competitive inhibitor of tyrosine hydroxylase, the rate-limiting enzyme involved in catecholamine synthesis (Nagatsu et al, 1964). Catecholamines, particularly dopamine, have been implicated in various aspects of reward processing including the ability to learn through reinforcement and to flexibly modify responses on the basis of changing reinforcement expectancies.
Impaired processing of reward-related stimuli and attentional bias toward negative information have been hypothesized to constitute behavioral endophenotypes in major depressive disorder (MDD) (Hasler et al, 2004). These behavioral deficits may reflect the biological endophenotype of reduced dopaminergic and noradrenergic function in depression (Hasler et al, 2008). To identify relationships between catecholamine function and potential deficits in reward learning as trait characteristics in MDD, we included subjects with MDD in full remission (RMDD) and healthy volunteers without increased risk for depression. We selected three tasks. The first two, the probabilistic response reversal (PRR) task (Budhani and Blair, 2005) and the passive avoidance (PA) learning task (Newman and Kosson, 1986) rely on positive outcome reinforcement signaling. Earlier work has shown that successful performance on both these tasks relies on the representation of reinforcement expectancy information by orbital frontal cortex (see Hampton et al, 2006; Kosson et al, 2006; Budhani et al, 2007). If CD disrupts the representation of reward expectancy information, it can be predicted that CD will disrupt performance on both tasks perhaps particularly in RMDD. The third task, the affective Stroop task (aSt) (Blair et al, 2007), assesses the degree to which emotional information interferes with the representation of task-relevant material. If CD interferes with top down attentional control (Coull, 1998), it can be predicted that CD will increase interference in the aSt, perhaps particularly in RMDD.
MATERIALS AND METHODS
Participants
Female right-handed individuals aged 18–56 years either met DSM-IV criteria for MDD in full remission (RMDD) or had no history of any psychiatric disorder and no major psychiatric condition in first-degree relatives. Diagnosis was established using the Structured Clinical Interview for DSM-IV (First et al, 2001) and confirmed by an unstructured interview with a psychiatrist. The educational level was scored as follows: 1=grade 6 or less; 2=grade 7–12; 3=graduating high school; 4=part college; 5=graduated 2 year college; 6=graduated 4 year college; 7=part graduate/professional school; 8=completed graduate/professional school. The subjects were recruited through the outpatient clinical services of the NIMH and by advertisements in local newspapers and posters on the NIH campus. Exclusion criteria included major medical illnesses, pregnancy, psychotropic drug exposure (including nicotine) within 3 months, substance abuse within 1 year, lifetime history of substance dependence, psychiatric disorders other than MDD, or structural brain abnormalities on MRI. Inclusion criteria required that RMDD subjects had remained in remission while off medications ⩾3 months, and manifested depression-onset before age 40 years. Written informed consent was obtained as approved by the NIMH IRB, and the study has been carried out in accordance with the Declaration of Helsinki.
Experimental Design
Using a randomized, double-blind, placebo-controlled, crossover-design, subjects underwent two identical sessions separated by at least 1 week, in which they received either AMPT or placebo. To reduce risk for adverse reactions we used a body weight-adjusted AMPT dose of 40 mg/kg body weight p.o., to a maximum of 4 g, over 22 h. Each session involved 3 days, performed on an inpatient basis at the NIH Clinical Center. To reduce the risk of crystalluria during AMPT administration, subjects received sodium bicarbonate, drank ⩾2 l of water daily, and underwent urine analysis twice daily.
Brain Imaging
Two hours before neuropsychological testing, resting cerebral glucose metabolism was assessed by means of positron emission tomography and [F-18]fluorodeoxyglucose. The methods of image acquisition and analysis including the selection and boundaries of the brain regions-of-interest are detailed in Hasler et al (2008). Exploratory correlational analysis examined the relationship between behavioral performance and metabolic activity changes following AMPT administration. In earlier work, we have shown that CD influenced metabolic activity in several neural regions (Hasler et al, 2008). These regions include those implicated earlier in successful reversal learning and/or PA; in particular, the perigenual anterior cingulate cortex, ventrolateral prefrontal cortex, amygdala, and ventral striatum (Budhani et al, 2007; Kosson et al, 2006). We thus examined whether the neurophysiological effect of CD, as measured by the change in regional metabolic activity (averaged across hemispheres) under AMPT vs placebo, is related to behavioral performance.
Neuropsychological Testing
The neuropsychological assessments were initiated 34 h after the first AMPT intake and included the PRR task, the PA learning task, and the aST. The order of the tasks was randomized across participants.
The PRR task was described earlier in Budhani and Blair (2005). The stimuli were 12 line drawings of animals (Snodgrass and Vanderwart, 1980) each shaded a different color. These stimuli were randomly assigned to pairs at the beginning of the task. Stimuli measured 4cm × 4 cm and were presented on a gray background.
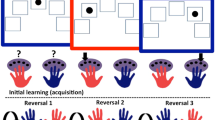
On each trial, one of the stimulus pairs was presented on a computer screen. The location of individual stimuli was randomly assigned to one of 16 locations on each trial. Participants chose one of the stimuli by clicking on it with the mouse, after which they received either positive (‘you win 100 points’) or negative (‘you lose 100 points’) feedback on the basis of the reinforcement contingency of that pair. A running total of points were presented at the bottom of the screen after each trial. Trials were self-paced. The reinforcement contingencies were probabilistic such that the ‘correct’ pair was not always rewarded and the ‘incorrect’ pair was not always punished. The ‘correct’ stimulus in a pair with an 80–20 reward–punishment contingency was rewarded on 8 out of every 10 trials and punished on 2 out of every 10 trials. Conversely, the ‘incorrect’ stimulus was punished on 8 out of every 10 trials and rewarded on 2 out of every 10 trials. The order of probabilistic feedback was randomized within the program. There were six different pairs of stimuli: two test pairs that changed contingency (reversing pairs) and four ‘dummy’ pairs that did not (nonreversing pairs). The two reversing pairs had contingencies 100−0 and 80−20. The reinforcement contingency of the reversing pairs remained constant for 40 trials (phase 1: acquisition of the discrimination). On completing 40 trials, the reinforcement contingency of the reversing pairs reversed (phase 2: reversal of the discrimination), so that the previously correct stimulus became the incorrect stimulus and the previously incorrect stimulus now became the correct stimulus. This reversed pattern continued for a total of 80 trials per stimulus pair. Three of the nonreversing dummy pairs had a contingency of 100−0 and the fourth had a contingency of 80−20.
The PA task was a modified version of Newman and Kosson's task (Newman and Kosson, 1986; Blair et al, 2004). Stimuli were 16 white two-digit numbers presented for 3000 ms sequentially on a black background. Six of the stimuli, the S+s were ‘good’ stimuli; an approach (bar press) response to these stimuli led to the participant gaining 100 points. Six of the stimuli, the S−s were ‘bad’ stimuli; the participant learned to avoid these stimuli as an approach (bar press) response to them led to the participant losing 100 points. Participants learned by trial-and-error to click on the mouse button to the S+ and to refrain from responding to the S−. After each response, participants received feedback on points they had won or lost. If no response was made, a blank screen appeared in place of feedback. Stimuli were presented once per block for 10 blocks per session. Performance was assessed by analysis of omission errors (failure to respond to a rewarded stimulus) or commission errors (response to a punished stimulus). The omission error rate was equal to the number of times a participant failed to respond to an S+ (and thus failed to obtain a reward). The PA error rate was defined as the number of times a participant responded to an S− (and was thus punished). Following earlier work (Newman and Kosson, 1986; Finger et al, 2007), the omission and PA data were analyzed with separate 2 (group: patients vs healthy comparison) × 2 (drug: AMPT vs placebo) × 10 (block) ANOVAs.
The aST (Blair et al, 2007) was adapted from a Number Stroop task developed by Pansky and Algom (2002). In the original Number Stroop task, participants are presented sequentially with two numerical displays presented within a nine-point grid (see Figure 1). The subject must determine which numerical display contains the greater numerosity. If there were more numbers in the first numerical display (50% of task trials), they responded by pressing a button with their left hand (more numbers in the second numerical display was instead indicated using a right-hand response). Participants did not receive feedback on their performance. The Stroop element of the task is based on the competition between the numerosity and number-reading information. On congruent trials, the Arabic numeral distracter information was consistent with the numerosity information; that is, the second (greater numerosity) display also contained Arabic numerals of larger value than the first display (eg two 2s and four 4s) (see Figure 1). On incongruent trials, the Arabic numeral distracter information was inconsistent with the numerosity information; that is, the second (greater numerosity) display contained numerals of smaller value than the first display (eg four 5s and five 4s) (see Figure 1). There were three different levels of incongruent trials according to the numerical distance between the numerosity and Arabic numeral information. Incongruent trials with a distance of 1 (two 3s and three 2s) are significantly more difficult than incongruent trials with a distance of 3 (two 5s and five 2s). The aST modifies this Number Stroop task by having positive, negative, or neutral images temporally bracket the numerical displays such that the trial consists of four, very rapid (400 ms each) consecutive displays (eg four 5s, picture of snake, five 4s, picture of snake). The emotional stimuli consisted of 40 positive, 40 negative (primarily threat related), and 40 neutral pictures selected from the International Affective Picture System (IAPS; Lang and Greenwald, 1988). The normative mean (±standard error (SE)) valence and arousal values on a nine-point scale were, respectively, 2.71±0.11 and 5.85±0.11 for negative pictures, 7.30±0.11 and 5.01±0.10 for positive pictures, and 4.96±0.07 and 2.78±0.08 for neutral pictures. Overall, each participant was presented with 480 trials (160 positive, 160 negative, and 160 neutral). Within each of the 160 trials, for each valence, 40 were congruent, 40 were incongruent distance 1, 40 were incongruent distance 2, and 40 were incongruent distance 3. Trials were randomized across participants.
Probabilistic response reversal task: number of errors by treatment and pair and learning phase. As expected, the numbers of errors were higher in the 100 : 0 pair trials than in the probabilistic 80 : 20 pair trials (p<0.001); and there were more errors in the reversal phase than in the acquisition phase (p<0.001). In the reversal phase of the 80 : 20 pair trials, errors were more frequent following catecholamine depletion than under placebo (drug by pair by phase interaction, p<0.05).
RESULTS
Of the 15 female RMDD subjects (mean age=39±11 years; HDRS<8 (mean=1.9±1.9), 3 had one earlier major depressive episode, 7 had two earlier episodes, and 5 had three or more earlier episodes. The 12 healthy female controls did not differ significantly from the RMDD subjects regarding mean age (mean age=39±12 years; mean HDRS=0.7±1.2). There was no difference in educational level between groups (mean educational level in RMDD subjects: 6.1±1.0; in controls: 6.3±0.62; p=0.70). The behavioral, neural, and endocrine responses to AMPT in the same study samples are described in Hasler et al (2008).
Probabilistic Reversal Learning Task
A 2 (group: patients vs healthy comparison) × 2 (drug: AMPT vs placebo) × 2 (pair: 100−0 vs 80−20) × 2 (phase: acquisition vs reversal) ANOVA was conducted on errors to criterion. This showed main effects for pair (F(1, 24)=24.35; p<0.001; mean errors 100−0 pair=1.59 (SE=0.16); mean errors 80−20=3.69 (SE=0.36)) and phase ((F(1, 25)=31.86; p<0.001; mean errors acquisition=1.49 (SE=0.25); mean errors reversal=3.80 (SE=0.36)). There were significant interactions for drug by pair (F(1, 25)=4.99; p<0.05), pair by phase (F(1, 25)=6.23; p<0.05), and, critically, drug by pair by phase (F(1, 25)=5.35; p<0.05; see Figure 1). As shown in Figure 1, AMPT selectively and significantly increased errors for the reversal of the 80−20 contingency pair (F(1, 27)=4.94; p<0.05). There was no significant main effect of, or interaction with, diagnosis (p>0.20 in all cases).
Passive Avoidance Learning
The ANOVA conducted on the omission error data showed both a main effect for block (F(9, 207)=4.04; p<0.005)) and a drug by block interaction (F(1, 23)=5.31; p<0.05; linear contrast); see Figure 2. This interaction was driven by the fact that under CD participants were less likely to respond to the S+ stimuli in the later blocks (7–10) relative to the earlier blocks (1–4) (F(1, 23)=10.29; p<0.01) whereas participants administered placebo were not (F(1, 23) <1; n.s.). There was no significant main effect of, or interactions with, diagnosis (p>0.15 in all cases). A second 2 (group: patients vs healthy comparison) × 2 (drug: AMPT vs placebo) × 10 (block) ANOVA was conducted on the PA error data. This showed no significant main effect of, or interaction with, drug (p>0.45 in all cases). However, there was a highly significant main effect for block (F(1, 23)=88.93; p<0.001); participants made fewer commission errors as the blocks progressed.
Passive avoidance learning task: number of omission of responses to rewarded stimuli by treatment and block. Following catecholamine depletion, subjects were less likely to respond to S+ stimuli in the later blocks (7–10) relative to the earlier blocks (1–4, p<0.01), whereas under placebo subject did not show such an influence of the blocks on the number of omission errors (drug by block interaction, p<0.05).
Affective Stroop task
Two 2 (group: patients vs healthy controls) × 2 (drug: AMPT vs placebo) × 3 (emotion: positive, negative, neutral) × 4 (distance: congruent, distance 3, distance 2, distance 1) ANOVAs were conducted on the RT and error data, respectively. The RT ANOVA showed main effects for emotion (F(2, 48)=16.73; p<0.001) and distance (F(3, 72)=16.69; p<0.001). The participants were slower to respond in the context of positive and negative distracters relative to neutral distracters (mean positive=889.8 ms (SE=30.65); mean negative=895.43 ms (SE=31.44); mean neutral=872.55 ms (SE=31.49)). The participants were slower to respond to the different distance incongruent trials relative to the congruent trials (mean RT for distance 1=893.73 ms (SE=30.63); mean for distance 2=901.61 ms (SE=30.38); mean for distance 3=895.01 ms (SE=31.70); mean congruent=852.21 ms (SE=33.11)). There was also a trend of drug (F(1, 24)=3.74; p<0.1), as participants were slower to respond under CD than under placebo (mean RT under CD=904.96 ms (SE=30.65); mean placebo=866.33 (SE=32.85)). There was no significant main effect of, or interaction with, diagnosis (p>0.10 in all cases).
The error rate ANOVA showed no significant main effect of drug or emotion (p=0.472 and 0.12, respectively). However, there was a main effect of distance (F(3, 72)=3.84; p<0.05). The participants made greater numbers of errors as numerical distance between the target and distracter information decreased (mean distance 1=0.68 (SE=0.13); mean distance 2=0.60 (SE=0.12); mean distance 3=0.55 (SE=0.10); mean congruent=0.39 (SE=0.08)). There was also a significant group by distance interaction (F(3, 72)=3.77; p<0.05). The RMDD individuals made significantly greater errors for distance 2 (F(1, 24)=5.08; p<0.05); see Figure 3).
Affective stroop task: error rates by group and condition. As expected, subjects made a greater number of errors as numerical distance between the target and distracter information decreased (p<0.05). In addition, the fully remitted subjects with MDD made significantly more errors for distance 2 (p<0.05; group by distance interaction, p<0.05).
Correlations With Brain Metabolism
Two measures were generated from the two reward learning tasks: (1) As CD selectively and significantly increased errors for the reversal of the 80−20 contingency pair, the first behavioral performance difference score was AMPT 80−20 reversal errors—Placebo 80−20 reversal errors; (2) As participants under CD significantly increased missed responses to the S+ ‘good’ stimuli in the later blocks relative to the earlier blocks, we generated the behavioral performance difference score: AMPT misses of good stimuli for blocks 7–10—AMPT misses of good stimuli for blocks 1–4. Thus, 10 correlations were conducted. These resulted in two clear results: the greater the extent to which metabolism in perigenual anterior cingulate cortex (ACC) decreased under CD (Figure 4), the greater the number of 80 : 20 reversal errors occurred under CD relative to placebo (r=−0.52; p<0.01) and the more often good stimuli were missed in blocks 7–10 relative to blocks 1–4 under CD vs placebo (r=−0.46; p<0.05).
Placement of the perigenual anterior cingulate cortex (ACC) region-of-interest (ROI) in the horizontal plane. The crosshair is placed over the pregenual ACC within the left perigenual ACC ROI. In this voxel, CD-induced change in brain metabolism correlated with CD-induced errors in the reward learning tasks: the greater the extent to which metabolism in perigenual ACC decreased under CD, the greater the number of 80 : 20 reversal errors occurred under CD relative to placebo (r=−0.52; p<0.01) and the more often good stimuli were missed in blocks 7–10 relative to blocks 1–4 under CD vs placebo (r=−0.46; p<0.05).
DISCUSSION
This is the first study examining the effects of CD on reversal learning and PA in humans. Although AMPT did not affect the acquisition phase of the reversal learning task, it selectively impaired reversal of the 80−20 contingency pair and reduced responding toward rewarding stimuli. Moreover, the performance decrement produced by AMPT on both tasks was associated with the level of decreased metabolism in the perigenual ACC. Finally, using the aST, we found evidence for impaired executive attention, reflected by a greater error rate in an executive attention task in RMDD than in controls, as a trait abnormality in MDD.
Dopamine and to a lesser extent norephinephrine have been implicated in various aspects of reinforcement-based learning (Crow and Wendlandt, 1976; Wilkinson et al, 1998; Kabai et al, 2004). Moreover, earlier studies have provided evidence for a prominent role of dopamine in reversal learning. For example, in mice administration of the selective D1-like agonist SKF81297 produced an impairment in the early phase of reversal learning (Izquierdo et al, 2006), administration of the D2/D3 receptor antagonist raclopride impaired performance in the reversal of a learned visual discrimination in monkeys (Lee et al, 2007); administration of amphetamine or cocaine, which increases intrasynaptic dopamine concentrations, impaired reversal learning and induced response perseveration (Ridley et al, 1981; Stalnaker et al, 2007). The literature is, however, in disagreement regarding the effects of reduced dopaminergic neurotransmission on reversal learning. Although dopaminergic lesions of the nucleus accumbens impaired reversal learning in rodents (Taghzouti et al, 1985), depletion of dopamine in the orbitofrontal cortex did not impair serial discrimination reversal learning in mice (Clarke et al, 2007), and dopaminergic antagonists such as haloperidol caused only a mild, nonperseverative impairment on reversal learning in marmosets (Ridley et al, 1981). The current data confirm the role of dopamine in reinforcement-based decision making and suggest that, in humans, dopamine depletion impairs reversal learning. Interestingly, enhanced dompaminergic activity has also been associated with impaired reversal learning: in Parkinson's patients, dopaminergic medication impaired probabilistic reversal learning, possibly because of ‘over-dosing’ of the ventral striatum, which is relatively spared of dopamine loss in early stage Parkinson's disease (Cools et al, 2001, 2007). Interactions between the dopaminergic and the serotonergic systems during reversal learning have been proposed because tryptophan depletion also affected reversal learning, particularly, during the processing of aversive signals by modulation of the dorsomedial PFC (Evers et al, 2005).
Studies of experimental animals indicate that performance on a task homologous to the current PA learning task relies on the amygdala, striatum, and orbitofrontal cortex (Schoenbaum et al, 2006). FMRI data confirm the role of these structures in humans examined during PA learning (Kosson et al, 2006). Studies in nonhuman primates have shown that a neural network that includes the orbitofrontal cortex, striatum, and ascending monoaminergic systems has a critical function in the ability to adjust responses during reversal learning (Iversen and Mishkin, 1970; Rolls et al, 1996; Clarke et al, 2004, 2007; Izquierdo et al, 2004; Bellebaum et al, 2008). These results have been extended to humans through fMRI studies (Hampton et al, 2006; Budhani et al, 2007). Thus, studies in humans and experimental animals implicate both orbitofrontal cortex and striatum in successful performance on both the PA and reversal learning tasks. Although metabolic activity changes within ventral striatum following AMPT were not related to performance decrements on these two tasks, metabolic changes within the perigenual ACC following AMPT were related to them. The perigenual ACC contains abundant concentrations of dopamine receptors, and its projections to the ventral tegmental area have major roles in organizing the release of dopamine in the striatum and prefrontal cortex (reviewed in Drevets et al, 1998). As such, the degree to which AMPT has an impact on metabolic activity within ACC may influence function in the orbitofrontal cortex and striatum, potentially accounting for the relationship between the change in ACC metabolism and the change in behavioral performance on two tasks that putatively rely on the function of the orbitofrontal cortex and striatum.
Both PA and reversal learning rely on positive outcome reinforcement signaling. Within the PA learning task, the subject must associate specific stimuli with reward and respond when they are present, while also associating other stimuli with punishment and avoid responding when they are present (Schoenbaum et al, 2006). Impaired representation of reinforcement outcome information thus will disrupt task performance. Within the reversal learning task, the subject must update reinforcement values associated with specific responses when the reinforcement contingencies change during the reversal phase (Hampton et al, 2006). The orbitofrontal cortex is critically involved in the representation of reinforcement outcomes (Hampton et al, 2006). Importantly, the current data add to the evidence of an association between dopamine (and possibly norepinephrine) neurotransmission and the representation of reinforcement outcome information. Brain signals related to reward-related learning have been located in the midbrain dopamine neurons, select neurons of the orbitofrontal and ventromedial prefrontal cortex, ventral and dorsal striatum, and amygdala (Everitt et al, 2003; O’Doherty, 2004; Schultz, 2007). In monkeys, a prominent relationship between oribtofrontal neuronal activity and outcome reinforcement signaling has been demonstrated (Tremblay and Schultz, 2000). Taken together, the mechanisms by which CD resulted in impairments of reversal learning and PA may involve effects on rapid dopamine phasic responses to reward-predicting stimuli within orbitofrontal and medial prefrontal cortex (Schultz, 1997). Although there has been little examination how coeruleo-cortical noradrenergic projections influence the processing of rewarding stimuli, it is possible that norephinephrine depletion may also have contributed to impaired reinforcement signaling. Alternatively, or additionally, there may have been an interaction effect of the dopamine and norepinephrine depletion by AMPT (Devoto et al, 2004).
Attentional bias toward processing of mood congruent information including sad, unpleasant, and negative words, and fearful and sad facial expression have been earlier reported in patients with MDD (Watkins et al, 1996; Murphy et al, 1999). Moreover, they have also been found in subjects with remitted MDD suggesting a trait-like abnormality (Hammen et al, 1985; Koschack et al, 2003). Although depletion of central serotonin led to the emergence of mood-congruent memory bias (Klaassen et al, 2002), the effects of CD on attentional and mnemonic biases toward negative information have not been examined. We found that both RMDD subjects and controls were slower to respond in the context of positive and negative distracters relative to neutral distracters, and they were slower to respond to the different distance incongruent trials relative to the congruent trials. AMPT led to a slight general increase in reaction time, but there was no interaction with diagnosis. The participants made greater numbers of errors as numerical distance between the target and distracter information decreased, and this effect was significantly more pronounced in RMDD subjects than controls. In summary, these findings suggest no important influence of catecholamines on attentional bias induced by emotional distractors and provide no evidence for attentional bias as a trait marker in MDD with respect to emotional distractors. However, this study confirms earlier reports of impairments of executive attention as assessed by the Stroop test as a neuropsychological trait in affective illness (Zubieta et al, 2001; Blumberg et al, 2003; Hasler et al, 2006).
Several limitations of our methods merit comment. We did not include an active placebo because of the pharmacological actions of sedatives (eg, anticholinergic or benzodiazepine agents) that have been used earlier as active controls in AMPT studies might have had affected task performance in the control condition and thus confounded the results of this study. Moreover, there was no difference between RMDD subjects and controls regarding the sedative effects of AMPT. The subject samples were small and included only female subjects, precluding generalization of the results to males. The generalizability of our results also was affected by selection biases introduced by the requirement that RMDD subjects had maintained remission while off medications for ⩾3 months, which yielded a sample with a relatively small number of past depressive episodes (2.5±1.5), which may have contributed to the lack of associations between AMPT-induced impairments of reward processing and risk of MDD. Finally, the sample size was relatively small for a behavioral study, which reduces the reliability of our results and calls for studies that evaluate their replicability.
In conclusion, this study showed specific effects of CD on the processing of reward-related stimuli in humans: CD impaired both the reversal of probabilistic contingency pairs and the retention of stimulus–reward learning in a PA task. These CD-induced impairments of reward processing were found both in healthy controls and in subjects with fully remitted MDD. In addition, this study confirms earlier investigations that show impairments of executive attention as a neuropsychological trait in affective illness (Hasler et al, 2006).
References
Bellebaum C, Koch B, Schwarz M, Daum I (2008). Focal basal ganglia lesions are associated with impairments in reward-based reversal learning. Brain 131: 829–841.
Berman RM, Narasimhan M, Miller HL, Anand A, Cappiello A, Oren DA et al (1999). Transient depressive relapse induced by catecholamine depletion: potential phenotypic vulnerability marker? Arch Gen Psychiatry 56: 395–403.
Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L et al (2007). Modulation of emotion by cognition and cognition by emotion. Neuroimage 35: 430–440.
Blair RJR, Mitchell DGV, Leonard A, Budhani S, Peschardt KS, Newman C (2004). Passive avoidance learning in psychopathic individuals: modulation by reward but not by punishment. Pers Individ Dif 37: 1179–1192.
Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC et al (2003). A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry 60: 601–609.
Budhani S, Blair RJ (2005). Response reversal and children with psychopathic tendencies: success is a function of salience of contingency change. J Child Psychol Psychiatry 46: 972–981.
Budhani S, Marsh AA, Pine DS, Blair RJ (2007). Neural correlates of response reversal: considering acquisition. Neuroimage 34: 1754–1765.
Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC (2004). Cognitive inflexibility after prefrontal serotonin depletion. Science 304: 878–880.
Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC (2007). Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex 17: 18–27.
Cools R, Barker RA, Sahakian BJ, Robbins TW (2001). Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex 11: 1136–1143.
Cools R, Lewis SJ, Clark L, Barker RA, Robbins TW (2007). L-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson's disease. Neuropsychopharmacology 32: 180–189.
Coull JT (1998). Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol 55: 343–361.
Crow TJ, Wendlandt S (1976). Impaired acquisition of a passive avoidance response after lesions induced in the locus coeruleus by 6-OH-dopamine. Nature 259: 42–44.
Devoto P, Flore G, Pira L, Longu G, Gessa GL (2004). Alpha2-adrenoceptor mediated co-release of dopamine and noradrenaline from noradrenergic neurons in the cerebral cortex. J Neurochem 88: 1003–1009.
Drevets WC, Ongur D, Price JL (1998). Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry 3: 220–226, 190–221.
Dunlop BW, Nemeroff CB (2007). The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64: 327–337.
Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW (2003). Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci 985: 233–250.
Evers EA, Cools R, Clark L, van der Veen FM, Jolles J, Sahakian BJ et al (2005). Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology 30: 1138–1147.
Finger EC, Marsh AA, Buzas B, Kamel N, Rhodes R, Vythilingham M et al (2007). The impact of tryptophan depletion and 5-HTTLPR genotype on passive avoidance and response reversal instrumental learning tasks. Neuropsychopharmacology 32: 206–215.
First MB, Spitzer RL, Gibbon M, Williams JBW (2001). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute: New York.
Hammen C, Marks T, Mayol A, deMayo R (1985). Depressive self-schemas, life stress, and vulnerability to depression. J Abnorm Psychol 94: 308–319.
Hampton AN, Bossaerts P, O’Doherty JP (2006). The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans. J Neurosci 26: 8360–8367.
Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK (2006). Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry 60: 93–105.
Hasler G, Drevets WC, Manji HK, Charney DS (2004). Discovering endophenotypes for major depression. Neuropsychopharmacology 29: 1765–1781.
Hasler G, Fromm S, Carlson PJ, Luckenbaugh DA, Waldeck T, Geraci M et al (2008). Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch Gen Psychiatry 65: 521–531.
Iversen SD, Mishkin M (1970). Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res 11: 376–386.
Izquierdo A, Suda RK, Murray EA (2004). Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci 24: 7540–7548.
Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM et al (2006). Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res 171: 181–188.
Kabai P, Stewart MG, Tarcali J, Csillag A (2004). Inhibiting effect of D1, but not D2 antagonist administered to the striatum on retention of passive avoidance in the chick. Neurobiol Learn Mem 81: 155–158.
Klaassen T, Riedel WJ, Deutz NE, Van Praag HM (2002). Mood congruent memory bias induced by tryptophan depletion. Psychol Med 32: 167–172.
Koschack J, Hoschel K, Irle E (2003). Differential impairments of facial affect priming in subjects with acute or partially remitted major depressive episodes. J Nerv Ment Dis 191: 175–181.
Kosson DS, Budhani S, Nakic M, Chen G, Saad ZS, Vythilingam M et al (2006). The role of the amygdala and rostral anterior cingulate in encoding expected outcomes during learning. Neuroimage 29: 1161–1172.
Lang PJ, Greenwald MK (1988). The International Affective Picture System Standardization Procedure and Initial Group Results for Affective Judgements. Center for Reseach in Psychophysiology, University of Florida: Gainesville, FL.
Lee B, Groman S, London ED, Jentsch JD (2007). Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology 32: 2125–2134.
Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW et al (1999). Emotional bias and inhibitory control processes in mania and depression. Psychol Med 29: 1307–1321.
Nagatsu T, Levitt M, Udenfriend S (1964). Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem 239: 2910–2917.
Newman JP, Kosson DS (1986). Passive avoidance learning in psychopathic and nonpsychopathic offenders. J Abnorm Psychol 95: 252–256.
O’Doherty JP (2004). Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol 14: 769–776.
Pansky A, Algom D (2002). Comparative judgment of numerosity and numerical magnitude: attention preempts automaticity. J Exp Psychol Learn Mem Cogn 28: 259–274.
Ridley RM, Haystead TA, Baker HF (1981). An analysis of visual object reversal learning in the marmoset after amphetamine and haloperidol. Pharmacol Biochem Behav 14: 345–351.
Rolls ET, Critchley HD, Mason R, Wakeman EA (1996). Orbitofrontal cortex neurons: role in olfactory and visual association learning. J Neurophysiol 75: 1970–1981.
Schoenbaum G, Setlow B, Saddoris MP, Gallagher M (2006). Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. J Neurophysiol 95: 1509–1517.
Schultz W (1997). Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol 7: 191–197.
Schultz W (2007). Multiple dopamine functions at different time courses. Annu Rev Neurosci 30: 259–288.
Snodgrass JG, Vanderwart M (1980). A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 6: 174–215.
Stalnaker TA, Roesch MR, Franz TM, Calu DJ, Singh T, Schoenbaum G (2007). Cocaine-induced decision-making deficits are mediated by miscoding in basolateral amygdala. Nat Neurosci 10: 949–951.
Taghzouti K, Louilot A, Herman JP, Le Moal M, Simon H (1985). Alternation behavior, spatial discrimination, and reversal disturbances following 6-hydroxydopamine lesions in the nucleus accumbens of the rat. Behav Neural Biol 44: 354–363.
Tremblay L, Schultz W (2000). Reward-related neuronal activity during go-nogo task performance in primate orbitofrontal cortex. J Neurophysiol 83: 1864–1876.
Volkow ND, Fowler JS, Wang GJ, Swanson JM (2004). Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 9: 557–569.
Watkins PC, Vache K, Verney SP, Muller S, Mathews A (1996). Unconscious mood-congruent memory bias in depression. J Abnorm Psychol 105: 34–41.
Wilkinson LS, Humby T, Killcross AS, Torres EM, Everitt BJ, Robbins TW (1998). Dissociations in dopamine release in medial prefrontal cortex and ventral striatum during the acquisition and extinction of classical aversive conditioning in the rat. Eur J Neurosci 10: 1019–1026.
Zubieta JK, Huguelet P, O’Neil RL, Giordani BJ (2001). Cognitive function in euthymic bipolar I disorder. Psychiatry Res 102: 9–20.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Mental Health. Compensation for professional services Dr Hasler: Eli Lilly, Switzerland; Servier, Switzerland. Krystal Mondillo: none. Dr Drevets: American Psychiatric Association. Dr Blair: none.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hasler, G., Mondillo, K., Drevets, W. et al. Impairments of Probabilistic Response Reversal and Passive Avoidance Following Catecholamine Depletion. Neuropsychopharmacol 34, 2691–2698 (2009). https://doi.org/10.1038/npp.2009.95
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2009.95
Keywords
This article is cited by
-
Presentation and Neurobiology of Anhedonia in Mood Disorders: Commonalities and Distinctions
Current Psychiatry Reports (2018)
-
To treat a psychopath
Theoretical Medicine and Bioethics (2014)
-
The neurobiology of psychopathic traits in youths
Nature Reviews Neuroscience (2013)
-
Nicotine withdrawal modulates frontal brain function during an affective Stroop task
Psychopharmacology (2012)