Abstract
Muscimol has been regarded as a universal agonist for all γ-aminobutyric acid type A receptor (GABAA-R) subtypes. However, brain regional distribution of muscimol's high-affinity binding sites greatly differs from those of other binding sites of the GABAA-R. To test whether behavioral effects of muscimol correlated with the density of high-affinity [3H]muscimol binding, we examined several GABAA-R subunit gene-modified mouse lines: α1, α4, or δ-knockouts (KO), α4+δ-double KO, and Thy1.2 promoter-driven α6 transgenic mice (Thy1α6). We determined the high-affinity [3H]muscimol binding in brain sections by quantitative autoradiography and sedative/ataxic effects induced in vivo by muscimol using a constant speed rotarod. α4-KO mice had reduced [3H]muscimol binding in the caudate-putamen, thalamus, and hippocampus, and were less sensitive to the behavioral impairment by muscimol. Similarly, δ-KO mice also had reduced binding to forebrain regions and a lower behavioral sensitivity to muscimol than their wild-type controls. In contrast, α1-KO mice had unaltered behavioral sensitivity to muscimol and unaltered [3H]muscimol binding, even though previous studies have demonstrated dramatically reduced binding to various other GABAA-R sites in these mice. Finally, Thy1α6 mice exhibited increased behavioral sensitivity to muscimol, and to another direct GABA-site agonist gaboxadol, and increased [3H]muscimol binding in the cerebral cortex and hippocampus. Thus, the differences in sedative and motor-impairing actions of muscimol in various mouse models correlated with the level of forebrain high-affinity [3H]muscimol binding. These data suggest that a small special population of GABAA-Rs, most likely extrasynaptic non-α1-containing receptors, strongly contributes to the in vivo pharmacological effects of muscimol.
Similar content being viewed by others
INTRODUCTION
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian central nervous system and it acts primarily through the GABA type A receptor (GABAA-R). GABAA-Rs are pentameric complexes that function as ligand-gated anion channels. They can be modulated by a number of clinically used sedative, hypnotic, and anesthetic drugs. There are a variety of subunit families that make up GABAA-Rs; a total of 19 distinct subunit genes have been cloned, α1–6, β1–3, γ1–3, δ, ɛ, π, θ, and ρ1–3 (Barnard et al, 1998). The subunit stoichiometry is usually two α-subunits+two β-subunits+one γ- or δ-subunit (Backus et al, 1993; Boileau et al, 2005). The diversity in GABAA-R subunit composition results in substantial, anatomical, functional, and pharmacological heterogeneity. For example, GABAA-Rs containing α1, α2, or α3, with β and γ2 are typically found at subsynaptic sites, where they mediate fast synaptic inhibition by synaptically released GABA and show potentiation by benzodiazepines, as these drugs bind to α/γ2 subunit interface (Sigel and Buhr, 1997). In contrast, GABAA-Rs containing α4 or α6, with β and δ are typically found at extrasynaptic or perisynaptic locations, where they mediate a tonic form of inhibition by virtue of their ability to respond to low concentrations of spill-over GABA and show insensitivity to classical benzodiazepines. Thus, receptors containing γ2 along with α1, α2, α3, or α5 are potentiated by benzodiazepines, whereas those containing α4 or α6 are not, but all receptor subtypes respond to GABA (Luddens and Wisden, 1991; Sieghart, 1995).
Muscimol, a constituent and psychoactive ingredient of the mushroom Amanita muscaria, is regarded as a compound that activates all GABAA-R subtypes (Krogsgaard-Larsen et al, 1979), and it is described as the prototypic exogenous agonist for GABAA-Rs in current textbooks (Meyer and Quenzer, 2005; Brunton et al, 2006). Thus, its effects on behavior and brain metabolism should be similar or at least comprise all those effects that allosteric benzodiazepines produce. In agreement, muscimol differs from benzodiazepines in its more global actions on brain metabolism; eg, muscimol reduces cerebral glucose metabolism more strongly than the benzodiazepine clonazepam that actually shows a ceiling effect at higher receptor occupancy (Ito et al, 1994). However, muscimol and benzodiazepines affect glucose metabolism in different brain regions (Brett and Pratt, 1991; Kelly et al, 1986; Kelly and McCulloch, 1982), and behavioral effects of muscimol and benzodiazepines are often different, even up to the level that benzodiazepines have been concluded to act through some other mechanism than facilitation of GABAA-R function (Mendelson and Monti, 1993). Spatial distribution of high-affinity [3H]muscimol binding at low nanomolar concentrations differs from that of benzodiazepine site ligands throughout the rodent brain (Korpi et al, 2002a; Mans et al, 1992; Olsen et al, 1990; Palacios et al, 1981). [3H]Muscimol and [3H]GABA bind to two kinds of agonist binding sites on the GABAA-Rs with three- to twenty-fold difference in affinity (Browner et al, 1981; Burch et al, 1983; Wang et al, 1979), with the binding site being formed at α/β-interfaces (Sigel and Buhr, 1997).
Biochemical and pharmacological experiments have suggested that high- and low-affinity conformations might be interchangeable forms of the same GABAA-R complex (see for discussion, Agey and Dunn, 1989; Maksay, 1996; Sieghart, 1995). Interestingly, in most studies, functional responses to GABA have been observed only at micromolar concentrations, as opposed to nanomolar concentrations that are needed to occupy the high-affinity binding sites, and, therefore, the high-affinity sites have been interpreted to represent a desensitized form of GABAA-R or otherwise non-functional binding sites (DeLorey and Brown, 1992; Dunn and Thuynsma, 1994; Edgar and Schwartz, 1992; Maconochie et al, 1994; Maloteaux et al, 1987; Mennini and Gobbi, 1990; Uusi-Oukari and Korpi, 1992; but see Birnir and Korpi, 2007). Recently, the high-affinity [3H]muscimol binding has been associated with α6- and δ-subunits of GABAA-R in the cerebellum and with δ-subunits in the forebrain (Korpi et al, 2002b; Mihalek et al, 1999; Quirk et al, 1995); ie, this pharmacological fingerprint might be specific to subtype(s) of GABAA-R not containing γ2-subunit.
To understand the possible behavioral significance of GABAA-Rs forming high-affinity [3H]muscimol binding sites, we used novel genetic mouse lines with known or predicted differences in forebrain high-affinity [3H]muscimol binding, and tested them for sedative/ataxic behavioral effects of muscimol. The mice either lack GABAA-R α1, α4, δ or α4 and δ-subunits (knockout models) or they ectopically overexpress the cerebellar α6-subunit in the forebrain (a transgenic model). Behavior was measured by fixed-speed rotarod test with muscimol, and brain regional high-affinity binding of [3H]muscimol was assessed by quantitative ligand autoradiography.
MATERIALS AND METHODS
Animals
Five different GABAA-R subunit mutant mouse strains were used: (1) α1-subunit knockout (KO) (Vicini et al, 2001, 2) α4-subunit KO (Chandra et al, 2006, 3) δ-subunit KO (Mihalek et al, 1999, 4) α4+δ-double KO, and (5) Thy1.2 promoter-driven α6-subunit transgenic mice (Wisden et al, 2002). Thy1α6 mice express ectopically extrasynaptic α6β±γ2 GABAA-R in the forebrain, especially in the hippocampus (Sinkkonen et al, 2004; Wisden et al, 2002), which results in increased tonic GABAA-R current in their hippocampal CA1 principal neurons. All KO mice were compared with their wild-type littermate controls and were created by heterozygous interbreeding. Genetic backgrounds of the single KO lines are as follows: α4-KO (mixed C57BL/6J and Strain 129S1/X1, F2-F6 generations), δ-KO (mixed C57BL/6J and Strain 129S1/X1, >F20 generations), and α1-KO (mixed C57BL/6J, FvB, and Strain 129S1/X1 from >F15 generations). α4+δ-double KOs were produced by mating the aforementioned α4-KO and δ-KO mice and interbreeding their double heterozygous offspring. Thy1α6 transgenic mice were on a C57BL/6 background (>10 generations; Saarelainen et al, 2008). Mice were produced for experiments by mating within a homozygous line, and compared with C57BL/6NHsd controls (Harlan Netherland, Horst, Netherlands), which were purchased at the age of 5 weeks and housed in the same facility until experiments took place.
At weaning, mice were genotyped using Southern blot analysis of tail DNA. Mice were group housed, given free access to standard rodent chow and water, and maintained on a 12-h alternating light/dark schedule with lights on at 0700 hours, with the temperature of 20–22°C. Autoradiographic experiments used mice at the age between 2 and 3 months. Behavioral experiments used both male and female drug-naive mice that were age matched and between 2 and 6 months old. Gender differences were observed in some cases (noted below). Wherever no gender differences were observed, data were pooled across gender.
The animal care and use committees of the Universities of Pittsburgh and Helsinki approved all experimental procedures.
Ligand Autoradiography
In the first set of autoradiography experiments, we compared the GABAA-R α4-KOs (n=3), δ-KOs (n=3), α4+δ-KOs (n=5), and their common wild-type controls (n=4); in the second set, the α1-KOs (n=5) and their wild-type littermate controls (n=6). In the last set, we compared the Thy1α6 (n=6) mice to their wild-type controls (n=6). Mice were decapitated, the brains were carefully dissected out, rinsed in ice-cold saline and frozen on dry ice. The frozen brains were wrapped airtight in plastic, and then stored at −80°C.
The autoradiographic procedures for GABA-sensitive high-affinity [3H]muscimol binding were as previously described in detail (Korpi et al, 2002a). Fourteen-μm-thick horizontal sections were cut with a Leica CM 3050 S cryostat, thaw-mounted onto gelatine-coated object glasses (Menzel GmbH, Braunsweig, Germany) and stored at −80°C until used in the experiments. For autoradiography, the sections were preincubated in an ice–water bath for 15 min in 0.17 M Tris-HCl (pH 7.4). Final incubations in the same buffer were performed with 15 nM [3H]muscimol (Perkin-Elmer, Boston, MA) at 0–4°C for 30 min. All binding signal was sensitive to 100 μM GABA (Sigma, St Louis, MO; data not shown). After incubation, the sections were quickly washed in ice-cold incubation buffer twice for 30 s. Sections were then dipped into distilled water, air-dried at room temperature, and exposed with plastic 3H-microscales standards (GE Healthcare, Little Chalfont, Buckinghamshire, UK) to Kodak Biomax MR films (Eastman Kodak, Rochester, NY) for 4 months.
Representative images from autoradiography films were scanned using an EPSON Expression 1680 Pro scanner and EPSON Scan v. 1.11e program and finalized in CorelDraw X3 (Corel Corporation, Ottawa, Canada). For quantification of binding densities, the films were first scanned with the standards and then analyzed with Scion Image analysis program (Scion Corporation, Frederick, Maryland). Binding densities for each brain area were referenced to the standards, converted to radioactivity levels estimated for gray matter areas (nCi/mg), and given as means±SE.
For each experimental set, one-way analysis of variance (ANOVA) and/or Bonferroni corrected t-test were used to assess the statistical significance of the differences using SPSS program (version 15.0; SPSS, Chicago, IL, USA).
Fixed Speed Rotarod
α1, α4, and δ-KO mice were tested using the Ugo Basile 7650 (Varese, Italy) apparatus with a rod diameter of 6 cm, rotating at a fixed speed of 6 r.p.m. Thy1α6 transgenic mice were tested on a Rotamex 4/8 (Columbus Instruments, Ohio, USA) with a rod diameter of 4 cm, rotating at 15 r.p.m. Mice were acclimated to the apparatus by pretraining them on the rotarod 1–3 times on the day before testing muscimol or gaboxadol. Only mice that were capable of walking on the rotarod for 180 s were used for drug-induced ataxia experiments. No difference in rotarod performance between mutant mice and their wild-type controls were observed during this training. Mice were evaluated once again before drug injection. Muscimol (Tocris-Cookson, Avonmouth, UK or Ellisville, MO, USA) or gaboxadol hydrochloride (4,5,6,7-tetrahydroisoazolo(5,4-c)pyridin-3-ol; THIP; H. Lundbeck A/S, Copenhagen, Denmark) was diluted in saline and administered into the intraperitoneal (i.p.) cavity in a volume of 10 ml per kg of body weight. Mice were then placed on the rotarod every 30 min before injection. The time a mouse was able to stay on the rotarod was recorded. Data were analyzed by repeated measures ANOVA.
Open Field
To test the effect of muscimol (0.75 mg/kg, i.p.) on exploratory locomotor activity, the mice were individually placed 30 min after drug administration on a novel arena (box with a 50 × 50-cm white floor and 50-cm-high gray walls) for 5 min. The animal was monitored and its movements were analyzed using a CCD video camera above the arena and EthoVision Color-Pro 3.0 software (Noldus Information Technology, Wageningen, The Netherlands).
RESULTS
High-Affinity [3H]Muscimol Binding in the Brain Sections of Mouse Models
Distribution of high-affinity [3H]muscimol binding in various brain regions in all control mouse lines using the autoradiographic assay preferentially followed the expression pattern of GABAA-R δ- and α6-subunits (Korpi et al, 2002b; Makela et al, 1997; Quirk et al, 1995; Wisden et al, 1992), being abundant in the cerebellar granule cell layer and thalamus, but absent, eg, in the brainstem (Figure 1, Table 1).
Regional distribution of 15-nM [3H]muscimol binding in representative horizontal brain sections from various mouse lines. (a) α4+δ-KO, α4-KO, and δ-KO compared with their wild-type (WT) brain images, (b) α1-KO and the corresponding WT images, and (c) the Thy1α6 and C57BL/6 WT control images. OB, olfactory bulb; CPu, caudate putamen; Ctx, cerebral cortex; Th, thalamus; Hi, hippocampus; Gr, granule cell layer of the cerebellum.
The wild-type brains differed from the α4, δ, and α4+δ-KO brains (F3,11=7.9, p<0.005 for the cerebral cortex; F3,11=126.3, p<0.001 for the caudate-putamen, F3,11=59.0, p<0.001 for the thalamus; F3,11=4.4, p<0.05 for the hippocampus, and F3,11=6.1, p<0.05 for the granule cell layer of cerebellum), except for the olfactory bulb (F3,9=0.3, p>0.05). In keeping with Korpi et al (2002b), we confirmed the reduced binding in the δ-KO brains, especially in the caudate-putamen and thalamus (Figure 1, Table 1). The binding levels in the caudate-putamen, thalamus, and hippocampus were also reduced in the α4-KO brains, and the greatest reductions were found in the double α4+δ-KO brains. Conversely, the binding in the cerebellar granule cell layer was significantly reduced only in the double KO brains, suggesting that the main differences in the high-affinity [3H]muscimol binding took place in the forebrain regions.
GABAA-R α1-subunit does not seem to be important for the high-affinity [3H]muscimol binding, as there was no significant reduction of the binding in the α1 KO brains as compared with the control brains in any brain region (F1,9 <0.8, p>0.05; Figure 1, Table 1). This contrasts strongly with the great reduction in ligand binding to the ion channel and benzodiazepine sites of GABAA-R of the α1-KO brains (Halonen et al, 2009; Kralic et al, 2002).
Transgenic Thy1α6 mice express the cerebellar granule cell-specific α6-subunit gene under pan-neuronal Thy1.2 promoter, especially in the cerebral cortex and hippocampus (Wisden et al, 2002). [3H]Muscimol binding appears to be markedly increased in these areas of the transgenic mice (Figure 1, Table 1), increasing to 155 and 256% of the values for the wild-type mice in the cerebral cortex (F1,10=14.7, p<0.005) and hippocampus (F1,10=19.3, p<0.005), respectively. This change in the binding contrasts to the unaltered binding of an ion channel ligand in the Thy1α6 mice (Saarelainen et al, 2008).
Decreased Behavioral Sensitivity to Muscimol in Both α4-KO and δ-KO Mice
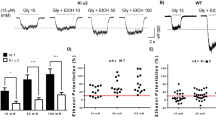
Recovery from ataxia induced by 1.5, 2.0, and 3.0 mg/kg muscimol was measured in α4-KO and control mice by fixed speed rotarod (Figure 2). α4-KO mice were significantly less sensitive to 1.5 mg/kg (Figure 2a; repeated measures ANOVA (F1,64=4.6, p<0.05)), 2.0 mg/kg (Figure 2b; F1,100=8.3, p<.05), and 3.0 mg/kg (Figure 2c; F1,100=6.1, p<0.05). There were no significant effects of gender (p>0.05) at any of the three doses and, therefore, the data from males and females were collapsed in the analysis. However, at 2.0 mg/kg, there was a trend toward a gender effect (F1,100=2.8, 0.05 < p<0.10), with females possibly being more sensitive to muscimol than males.
α4-KO mice have reduced sensitivity to the behavioral effects of muscimol. The fixed speed rotarod measured effects of muscimol on motor performance at 1.5 mg/kg (n=7 KO and 11 WT) (a), 2.0 mg/kg (n=12 KO and 15 WT) (b), and 3.0 mg/kg (n=12 KO and 15 WT) (c). Effect of muscimol was reduced in α4-KO mice (white squares) compared with WT mice (black squares) at 1.5 (p<0.05), 2.0 (p<0.05), and 3.0 mg/kg (p<0.05) (repeated measures ANOVA).
In the analysis of the δ-KO mice, there was a significant gender effect at the muscimol dose of 1.5 mg/kg (F1,45=4.9, p<0.05). Analysis of data split between genders revealed a significant effect of genotype in female mice (Figure 3a; p<0.01) but not in male mice (Figure 3b). Neither δ-KO nor wild-type males were affected by 1.5 mg/kg muscimol. At 2.0 and 3.0 mg/kg, no gender effect was observed. δ-KO mice recovered faster than the wild-type mice, following 2.0 mg/kg (Figure 3c; F1,104=17.8, p<0.001) and 3.0 mg/kg (Figure 3d; F1,104=21.5, p<0.0001).
δ-KO mice have reduced sensitivity to the behavioral effects of muscimol. The fixed speed rotarod measured effects of muscimol on motor performance in δ-KO mice (white squares) and WT mice (black squares). At 1.5 mg/kg, there was a significant effect of gender (p<0.05) and therefore, data were split between (a) females and (b) males. Motor impairment by the dose of 1.5 mg/kg in female δ-KO mice was greatly reduced (p<0.01) compared with female WT mice (n=5 KO and 5 WT). Male mice were not affected by muscimol at this dose (n=3 KO and 4 WT). δ-KO mice were less sensitive to the effects of muscimol at (c) 2.0 mg/kg (p<0.001; n=16 KO and 12 WT) and (d) 3.0 mg/kg (p<0.0001; n=16 KO and 12 WT) (repeated measures ANOVA).
These data demonstrate that both α4-KO and δ-KO mice are less sensitive to the ataxic effects of muscimol than the wild-type mice.
Unchanged Behavioral Sensitivity to Muscimol in α1-KO Mice
Muscimol-induced ataxia was assessed in α1-KO mice and their wild-type littermate controls. Recovery from ataxia induced by 2.0 (Figure 4a) and 3.0 mg/kg (Figure 4b) muscimol was not different between α1-KO and wild-type mice.
α1-KO mice are equally sensitive as WT mice to the behavioral effects of muscimol. The fixed speed rotarod measured effects of muscimol on motor performance in α1-KO mice (white squares) and WT littermate controls (black squares). No differences in genotypes were observed at 2.0 mg/kg ((a), n=10 KO and 12 WT) or 3.0 mg/kg ((b), n=10 KO and 12 WT) (repeated measures ANOVA).
Behavioral Supersensitivity to Muscimol in Thy1α6 Transgenic Mice
Ataxia induced by 1.0 or 1.5 mg/kg muscimol was compared between Thy1α6 transgenic mice and C57BL/6 wild-type control mice (Figure 5). There were significant effects of gender at both 1.0 mg/kg (F2,117=17.69, p<0.001) and 1.5 mg/kg (F2,117=2.67, p<0.05). Therefore, analysis of data was split by gender. At both doses and between both genders, Thy1α6 mice were more sensitive to muscimol than the control mice (Figure 5c: 1.0 mg/kg, females, F1,58=25.76, p<0.001; Figure 5d: 1.0 mg/kg, males, F1,58=10.51, p<0.001; Figure 5e: 1.5 mg/kg, females, F1,58=2.79, p<0.05; Figure 5f: 1.5 mg/kg, males, F1,58=3.72, p<0.05).
Thy1α6 transgenic mice have increased sensitivity to the ataxic effects of muscimol. The fixed speed rotarod measured the ataxic effects of 0.75, 1.0, or 1.5 mg/kg muscimol in Thy1α6 transgenic mice (white squares) and C57BL/6 WT controls (black squares). Muscimol impaired the performance of neither mouse line at the dose of 0.75 mg/kg (a, b). Because of significant differences between genders at the two higher doses, data were split between females and males. At those doses and between both genders, Thy1α6 mice were more sensitive to muscimol than controls ((c), females, 1.0 mg/kg, p<0.001; (d), males 1.0 mg/kg, p<0.001; (e), females, 1.5 mg/kg, p<0.05; (f), males, 1.5 mg/kg, p<0.05) (repeated measures ANOVA). n=6 per gender and genotype.
To test a lower dose, which had no significant effect on rotarod performance (Figure 5a and b), we used an open field test and determined exploratory locomotor activity after 0.75 mg/kg muscimol (Figure 6). Thy1α6 mice were more sensitive to muscimol than the control mice, as muscimol increased their total movements (Figure 6a, genotype × drug interaction F1,36=12.62, p<0.01) and the time they spent in the periphery (10-cm zone from the wall) (Figure 6b, interaction F1,36=24.86, p<0.001), and reduced the number of rears (Figure 6c, interaction F1,36=7.49, p<0.05).
Thy1α6 transgenic mice have increased sensitivity to the locomotor stimulating effect of muscimol in the open field test. Male Thy1α6 transgenic and C57BL/6 WT control mice were treated with saline or 0.75 mg/kg muscimol 30 min before being transferred to an open arena. Total locomotor activity (a), time spent in the periphery of the arena (b), and the number of rears (c) were determined for 5 min. Locomotor activity was increased by muscimol in Thy1α6 mice as compared with saline-treated mice (p<0.001) and muscimol-treated WT mice (p<0.001), especially in the periphery of the arena. Muscimol treatment reduced the number of rears in Thy1α6 mice (p<0.01). (Two-way ANOVA followed by Newman–Keuls post hoc test). n=10 males per genotype and treatment.
To test whether Thy1α6 mice are more sensitive to gaboxadol in the fixed-speed rotarod test, we administered a slightly sedating dose of gaboxadol (6 mg/kg) and determined the latency of falling from the rod (Figure 7). The transgenic mice were significantly more sensitive to gaboxadol than the wild-type controls (genotype effect F1,117=58.55, p<0.001).
Thy1α6 transgenic mice have increased sensitivity to the ataxic effects of the GABA-site agonist gaboxadol. The fixed speed rotarod measured the ataxic effects of 6.0 mg/kg gaboxadol in Thy1α6 transgenic mice (white squares) and C57BL/6 WT controls (black squares). There were no significant differences between the genders. Thy1α6 mice were more sensitive to muscimol than controls (p<0.001) (repeated measures ANOVA). n=6 per gender and genotype.
DISCUSSION
The present data using novel mouse models suggest that the prototypic direct GABAA-R agonist muscimol preferentially acts through high-affinity GABA sites. Ataxic/sedative responses to muscimol were measured using the rotarod. We observed that α4 or δ-KO mice, but not α1-KO mice, were less sensitive to muscimol-induced impairment, and in Thy1α6 mice, the sedative/ataxic and locomotor stimulating responses of muscimol were significantly increased compared with the wild-type mice. Importantly, these bidirectional behavioral differences from the corresponding wild-type mice could be correlated with the bidirectional alterations in density of high-affinity [3H]muscimol binding sites in the forebrain regions, such as the cerebral cortex, hippocampus, caudate-putamen, and thalamus of the gene-modified mice. Importantly, we did not find any consistent alterations in the cerebellar high-affinity [3H]muscimol binding in the mouse models, although the rotarod performance can be affected by selective modulation of the cerebellar circuits (Wulff et al, 2007).
Our finding is striking particularly if we consider that the α1-KO mice with unchanged high-affinity [3H]muscimol binding in brain sections have dramatic overall changes in their GABAA-Rs: (1) a 55% reduction in total GABAA-Rs, (2) a 35% reduction in muscimol-stimulated chloride uptake in cortical neurosynaptosomes, and (3) a 55% reduction in [3H]muscimol binding in cerebellar homogenates (Kralic et al, 2002). α1-subunit-containing GABAA-Rs are widely expressed throughout the brain, in far greater number than GABAA-Rs containing α4- or δ-subunits (Bencsits et al, 1999; Fritschy et al, 1992; Pirker et al, 2000; Quirk et al, 1995). It should be also noted that autoradiography showed no differences in high-affinity [3H]muscimol binding in a conditional α1-knockout mouse model (Sonner et al, 2005). Therefore, the behavioral differences we observed in other mouse models in response to muscimol very likely implicate the high-affinity non-α1 subunit-containing GABAA-Rs.
Reduced sensitivity to muscimol in α4-KO and δ-KO mice is consistent with in vitro studies of GABAA-Rs and molecular pharmacological studies using these KO lines. Muscimol has a 40% greater maximal effect on extrasynaptic GABAA-Rs than on synaptic GABAA-Rs (Ebert et al, 1997; Storustovu and Ebert, 2006). In addition, δ-KO mice have a greatly reduced number of high-affinity [3H]muscimol sites as measured by ligand autoradiography (Mihalek et al, 1999; this study). A similar reduction in the forebrain was also observed in α4-KO mice (this study). Therefore, reduced behavioral sensitivity of α4-KO and δ-KO mice may be because of the loss of the high-affinity [3H]muscimol binding sites.
Muscimol is structurally similar to GABA and has been used extensively as a lead compound for the design of several other GABA analogs, such as gaboxadol (Krogsgaard-Larsen et al, 2004). It is important to notice that changes in sensitivity to muscimol of transgenic mouse lines resemble closely the changes seen in sensitivity to gaboxadol, which mediates its action mainly directly on GABA sites of extrasynaptic receptors (Belelli et al, 2005; Jia et al, 2005). α4 and δ-KO mice are significantly less sensitive to gaboxadol (Boehm et al, 2006; Chandra et al, 2006), α1-KO mice have unaltered response to it (Herd et al, 2009) and Thy1α6 mice display increased sensitivity to it (Saarelainen et al, 2008). The regional distribution of high-affinity [3H]gaboxadol binding in the rat brain recapitulates that of [3H]muscimol binding (Friemel et al, 2007), both of them being very different from low-affinity GABAA-Rs detected, eg, by GABA-stimulation of benzodiazepine agonist binding (Mennini and Gobbi, 1990). This is consistent with gaboxadol and benzodiazepine agonists acting on behavior by different mechanisms and showing little cross-tolerance (Michelsen et al, 2007; Voss et al, 2003). Interestingly, the present findings suggest that at least some of the gaboxadol-induced behavioral effects may not be specific for that compound, but may be general properties of directly acting GABAA-R agonists. This idea was supported in this study by enhanced sensitivity to ataxic/sedative effects of both muscimol and gaboxadol in Thy1α6 mice (Figures 5 and 7). However, even if muscimol seems to show preferential behavioral activity through its high-affinity binding sites, our data demonstrate that residual effectiveness can be observed in our ataxia/sedation test by higher muscimol doses even in mouse lines with reduced high-affinity binding. This suggests that muscimol is a prototypic GABAA-R agonist with preferential efficacy and selectivity, but not specificity, to high-affinity receptor subtypes.
No pharmacokinetic data on muscimol are available at present for mice. In rats, it has been estimated using intravenous administration of [3H]muscimol (1 mg/kg) that, although it is quickly metabolized, the drug passes into the brain, resulting in a peak brain concentration of about 200 nmol/kg 30 min after administration (Baraldi et al, 1979; Moroni et al, 1982). Therefore, it is conceivable that in this study the concentrations achieved after i.p. dosing of 0.75–3.0 mg/kg muscimol would be remaining only at nanomolar levels. Thus, also the brain levels would be consistent with preferential action on high-affinity extrasynaptic vs low-affinity synaptic GABAA-Rs.
The exact subunit composition of GABAA-Rs mediating the high-affinity [3H]muscimol binding in autoradiography and the ataxic/sedative effect of muscimol remains to be solved in later studies. Biochemically, low- and high-affinity binding sites can be selectively and independently interconversed by chaotropic agents or chemicals modifying histidine or tyrosine residues (Browner et al, 1981; Burch et al, 1983; Maksay and Ticku, 1984). Presently, it is not known whether the sites recognized by [3H]muscimol in brain sections could be transformed to low-affinity binding sites. In recombinant α1β2γ2 GABAA-Rs, there are two agonist binding sites in one GABAA-R, both having different agonist binding properties (Baumann et al, 2003; Baur and Sigel, 2003). Specific amino-acid residues in the N-terminal extracellular domains of various α (and β) subunits are responsible for agonist binding and for differences in binding affinity and functional sensitivity between recombinant receptor subtypes (Amin and Weiss, 1993; Baur and Sigel, 2003; Bohme et al, 2004; Boileau et al, 1999, 2002). The agonist binding sites are most likely between α- and β-subunits. Because of the pentameric structure of GABAA-Rs, these two agonist binding sites are not symmetrically positioned, which forms basis to their functional difference. The fact, that a significant proportion of GABAA-Rs contain two different α-subunits (Benke et al, 2004) makes the number of possible agonist binding sites with different properties even larger. However, all recombinant GABAA-Rs display high-affinity agonist binding, and the difference in agonist binding affinity even between αβδ and αβγ2-subunit-containing GABAA-Rs has been modest (Hevers and Luddens, 1998; You and Dunn, 2007). It has been suggested that also the δ-subunit participates in the formation of agonist binding site (Kaur et al, 2009), eg, a domain containing the transmembrane regions 1 and 2 of the δ-subunit explains at least partly the high efficacy of various agonists, such as gaboxadol, in αβδ GABAA-Rs (You and Dunn, 2007). These molecular structural and functional data together with the data on mouse models (see above) make it very likely that the amino-acid compositions of various subunits, rather than posttranslational modifications, have the most important role in defining the agonist sensitivity. There still remains the mismatch between the autoradiographic signal for nanomolar [3H]muscimol binding and the rather similar agonist binding affinities of various recombinant ‘synaptic’ and ‘extrasynaptic’ GABAA-Rs. Part of that difference might be explained, eg, by the effects of auxiliary proteins in neurons (Everitt et al, 2004; see for review, Birnir and Korpi, 2007).
In conclusion, the present results point to a correlation between high-affinity agonist binding sites of the GABAA-R in the forebrain and behavioral sensitivity to muscimol, and thus suggest that the behavioral effects of muscimol are preferentially mediated through high-affinity agonist binding sites of the forebrain GABAA-Rs. Most likely these receptors are non-α1 extrasynaptic GABAA-Rs containing δ and α4-subunits. It should be noted that muscimol is not a specific agonist for these receptor subtypes, as at higher doses it was effective even in the absence of δ or α4-subunits. Importantly, the experimental transgenic model on ectopic upregulation of extrasynaptic α6-subunit-containing GABAA-Rs in the forebrain provided evidence for a correlation between increased high-affinity binding and increased behavioral effects of GABA-site agonists. Further dissection of GABAA-Rs to synaptic and extrasynaptic receptors mediating phasic and tonic inhibition, respectively, might enable pharmacological manipulation of different components of inhibitory circuits. In addition to molecular and pharmacological profiling of these specific GABAA-R populations, their physiological roles and behavioral effects caused by their manipulation need to be further studied.
References
Agey MW, Dunn SM (1989). Kinetics of [3H]muscimol binding to the GABAA receptor in bovine brain membranes. Biochemistry 28: 4200–4208.
Amin J, Weiss DS (1993). GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature 366: 565–569.
Backus KH, Arigoni M, Drescher U, Scheurer L, Malherbe P, Mohler H et al (1993). Stoichiometry of a recombinant GABAA receptor deduced from mutation-induced rectification. Neuroreport 5: 285–288.
Baraldi M, Grandison L, Guidotti A (1979). Distribution and metabolism of muscimol in the brain and other tissues of the rat. Neuropharmacology 18: 57–62.
Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G et al (1998). International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50: 291–313.
Baumann SW, Baur R, Sigel E (2003). Individual properties of the two functional agonist sites in GABAA receptors. J Neurosci 23: 11158–11166.
Baur R, Sigel E (2003). On high- and low-affinity agonist sites in GABAA receptors. J Neurochem 87: 325–332.
Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ (2005). Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J Neurosci 25: 11513–11520.
Bencsits E, Ebert V, Tretter V, Sieghart W (1999). A significant part of native γ-aminobutyric acidA receptors containing α4 subunits do not contain γ or δ subunits. J Biol Chem 274: 19613–19616.
Benke D, Fakitsas P, Roggenmoser C, Michel C, Rudolph U, Mohler H (2004). Analysis of the presence and abundance of GABAA receptors containing two different types of α subunits in murine brain using point-mutated α subunits. J Biol Chem 279: 43654–43660.
Birnir B, Korpi ER (2007). The impact of sub-cellular location and intracellular neuronal proteins on properties of GABAA receptors. Curr Pharm Des 13: 3169–3177.
Boehm SL, Homanics GE, Blednov YA, Harris RA (2006). δ-subunit containing GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridin-3-d. Eur J Pharmacol 541: 158–162.
Bohme I, Rabe H, Luddens H (2004). Four amino acids in the α subunits determine the γ-aminobutyric acid sensitivities of GABAA receptor subtypes. J Biol Chem 279: 35193–35200.
Boileau AJ, Evers AR, Davis AF, Czajkowski C (1999). Mapping the agonist binding site of the GABAA receptor: evidence for a β-strand. J Neurosci 19: 4847–4854.
Boileau AJ, Newell JG, Czajkowski C (2002). GABAA receptor β2 Tyr97 and Leu99 line the GABA-binding site. Insights into mechanisms of agonist and antagonist actions. J Biol Chem 277: 2931–2937.
Boileau AJ, Pearce RA, Czajkowski C (2005). Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABAA receptor-associated protein. J Neurosci 25: 11219–11230.
Brett RR, Pratt JA (1991). Muscimol-associated changes in local cerebral glucose use following chronic diazepam administration. Brain Res 558: 280–288.
Browner M, Ferkany JW, Enna SJ (1981). Biochemical identification of pharmacologically and functionally distinct GABA receptors in rat brain. J Neurosci 1: 514–518.
Brunton LL, Lazo JS, Parker KLE (2006). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th edn. McGraw-Hill: USA.
Burch TP, Thyagarajan R, Ticku MK (1983). Group-selective reagent modification of the benzodiazepine-γ-aminobutyric acid receptor-ionophore complex reveals that low-affinity γ-aminobutyric acid receptors stimulate benzodiazepine binding. Mol Pharmacol 23: 52–59.
Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF et al (2006). GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA 103: 15230–15235.
DeLorey TM, Brown GB (1992). γ-Aminobutyric acidA receptor pharmacology in rat cerebral cortical synaptoneurosomes. J Neurochem 58: 2162–2169.
Dunn SM, Thuynsma RP (1994). Reconstitution of purified GABAA receptors: ligand binding and chloride transporting properties. Biochemistry 33: 755–763.
Ebert B, Thompson SA, Saounatsou K, McKernan R, Krogsgaard-Larsen P, Wafford KA (1997). Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human γ-aminobutyric acid type A receptors. Mol Pharmacol 52: 1150–1156.
Edgar PP, Schwartz RD (1992). Functionally relevant γ-aminobutyric acidA receptors: equivalence between receptor affinity (Kd) and potency (EC50)? Mol Pharmacol 41: 1124–1129.
Everitt AB, Luu T, Cromer B, Tierney ML, Birnir B, Olsen RW et al (2004). Conductance of recombinant GABAA channels is increased in cells co-expressing GABAA receptor-associated protein. J Biol Chem 279: 21701–21706.
Friemel A, Ebert B, Hutson PH, Brust P, Nieber K, Deuther-Conrad W (2007). Postnatal development and kinetics of [3H]gaboxadol binding in rat brain: in vitro homogenate binding and quantitative autoradiography. Brain Res 1170: 39–47.
Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Mohler H (1992). Five subtypes of type A γ-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci USA 89: 6726–6730.
Halonen LM, Sinkkonen ST, Chandra D, Homanics GE, Korpi ER (2009). Brain regional distribution of GABAA receptors exhibiting atypical GABA agonism: roles of receptor subunits. Neurochem Int 55: 389–396.
Herd MB, Foister N, Chandra D, Peden DR, Homanics GE, Brown VJ et al (2009). Inhibition of thalamic excitability by 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3-ol: a selective role for δ-GABAA receptors. Eur J Neurosci 29: 1177–1187.
Hevers W, Luddens H (1998). The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol 18: 35–86.
Ito K, Sawada Y, Sugiyama Y, Suzuki H, Hanano M, Iga T (1994). Linear relationship between GABAA receptor occupancy of muscimol and glucose metabolic response in the conscious mouse brain. Drug Metab Dispos 22: 50–54.
Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA (2005). An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol 94: 4491–4501.
Kaur KH, Baur R, Sigel E (2009). Unanticipated structural and functional properties of δ-subunit-containing GABAA receptors. J Biol Chem 284: 7889–7896.
Kelly PA, McCulloch J (1982). Effects of the putative GABAergic agonists, muscimol and THIP, upon local cerebral glucose utilisation. J Neurochem 39: 613–624.
Kelly PA, Ford I, McCulloch J (1986). The effect of diazepam upon local cerebral glucose use in the conscious rat. Neuroscience 19: 257–265.
Korpi ER, Grunder G, Luddens H (2002a). Drug interactions at GABAA receptors. Prog Neurobiol 67: 113–159.
Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE et al (2002b). Altered receptor subtypes in the forebrain of GABAA receptor δ subunit-deficient mice: recruitment of γ2 subunits. Neuroscience 109: 733–743.
Kralic JE, Korpi ER, O′Buckley TK, Homanics GE, Morrow AL (2002). Molecular and pharmacological characterization of GABAA receptor α1 subunit knockout mice. J Pharmacol Exp Ther 302: 1037–1045.
Krogsgaard-Larsen P, Frolund B, Liljefors T, Ebert B (2004). GABAA agonists and partial agonists: THIP (Gaboxadol) as a non-opioid analgesic and a novel type of hypnotic. Biochem Pharmacol 68: 1573–1580.
Krogsgaard-Larsen P, Hjeds H, Curtis DR, Lodge D, Johnston GA (1979). Dihydromuscimol, thiomuscimol and related heterocyclic compounds as GABA analogues. J Neurochem 32: 1717–1724.
Luddens H, Wisden W (1991). Function and pharmacology of multiple GABAA receptor subunits. Trends Pharmacol Sci 12: 49–51.
Maconochie DJ, Zempel JM, Steinbach JH (1994). How quickly can GABAA receptors open? Neuron 12: 61–71.
Makela R, Uusi-Oukari M, Homanics GE, Quinlan JJ, Firestone LL, Wisden W et al (1997). Cerebellar γ-aminobutyric acid type A receptors: pharmacological subtypes revealed by mutant mouse lines. Mol Pharmacol 52: 380–388.
Maksay G (1996). From kinetics and thermodynamics of GABAA receptor binding to ionophore function. Neurochem Int 29: 361–370.
Maksay G, Ticku MK (1984). Characterization of γ-aminobutyric acid-benzodiazepine receptor complexes by protection against inactivation by group-specific reagents. J Neurochem 42: 1715–1727.
Maloteaux JM, Octave JN, Gossuin A, Laterre C, Trouet A (1987). GABA induces down-regulation of the benzodiazepine-GABA receptor complex in the rat cultured neurons. Eur J Pharmacol 144: 173–183.
Mans AM, Kukulka KM, McAvoy KJ, Rokosz NC (1992). Regional distribution and kinetics of three sites on the GABAA receptor: lack of effect of portacaval shunting. J Cereb Blood Flow Metab 12: 334–346.
Mendelson WB, Monti D (1993). Do benzodiazepines induce sleep by a GABAergic mechanism? Life Sci 53: PL81–PL87.
Mennini T, Gobbi M (1990). Regional distribution of low-affinity GABA receptors coupled to benzodiazepine receptor subtypes in rat brain: an autoradiographic evaluation. Eur J Pharmacol 189: 143–148.
Meyer JS, Quenzer LF (2005). Psychopharmacology—Drugs, the Brain, and Behavior. Sinauer Associates: Sunderland, MA, USA.
Michelsen S, Sanchez C, Ebert B (2007). Lack of generalisation between the GABAA receptor agonist, gaboxadol, and allosteric modulators of the benzodiazepine binding site in the rat drug discrimination procedure. Psychopharmacology (Berl) 193: 151–157.
Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP et al (1999). Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor δ subunit knockout mice. Proc Natl Acad Sci USA 96: 12905–12910.
Moroni F, Forchetti MC, Krogsgaard-Larsen P, Guidotti A (1982). Relative disposition of the GABA agonists THIP and muscimol in the brain of the rat. J Pharm Pharmacol 34: 676–678.
Olsen RW, McCabe RT, Wamsley JK (1990). GABAA receptor subtypes: autoradiographic comparison of GABA, benzodiazepine, and convulsant binding sites in the rat central nervous system. J Chem Neuroanat 3: 59–76.
Palacios JM, Wamsley JK, Kuhar MJ (1981). High affinity GABA receptors-autoradiographic localization. Brain Res 222: 285–307.
Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G (2000). GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850.
Quirk K, Whiting PJ, Ragan CI, McKernan RM (1995). Characterisation of δ-subunit containing GABAA receptors from rat brain. Eur J Pharmacol 290: 175–181.
Saarelainen KS, Ranna M, Rabe H, Sinkkonen ST, Moykkynen T, Uusi-Oukari M et al (2008). Enhanced behavioral sensitivity to the competitive GABA agonist, gaboxadol, in transgenic mice over-expressing hippocampal extrasynaptic α6β GABAA receptors. J Neurochem 105: 338–350.
Sieghart W (1995). Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev 47: 181–234.
Sigel E, Buhr A (1997). The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci 18: 425–429.
Sinkkonen ST, Vekovischeva OY, Moykkynen T, Ogris W, Sieghart W, Wisden W et al (2004). Behavioural correlates of an altered balance between synaptic and extrasynaptic GABAAergic inhibition in a mouse model. Eur J Neurosci 20: 2168–2178.
Sonner JM, Cascio M, Xing Y, Fanselow MS, Kralic JE, Morrow AL et al (2005). Alpha1 subunit-containing GABA type A receptors in forebrain contribute to the effect of inhaled anesthetics on conditioned fear. Mol Pharmacol 68: 61–68.
Storustovu SI, Ebert B (2006). Pharmacological characterization of agonists at δ-containing GABA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther 316: 1351–1359.
Uusi-Oukari M, Korpi ER (1992). Functional properties of GABAA receptors in two rat lines selected for high and low alcohol sensitivity. Alcohol 9: 261–269.
Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE (2001). GABAA receptor α1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci 21: 3009–3016.
Voss J, Sanchez C, Michelsen S, Ebert B (2003). Rotarod studies in the rat of the GABAA receptor agonist gaboxadol: lack of ethanol potentiation and benzodiazepine cross-tolerance. Eur J Pharmacol 482: 215–222.
Wang YJ, Salvaterra P, Roberts E (1979). Characterization of [3H]muscimol binding to mouse brain membranes. Biochem Pharmacol 28: 1123–1128.
Wisden W, Cope D, Klausberger T, Hauer B, Sinkkonen ST, Tretter V et al (2002). Ectopic expression of the GABAA receptor α6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology 43: 530–549.
Wisden W, Laurie DJ, Monyer H, Seeburg PH (1992). The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12: 1040–1062.
Wulff P, Goetz T, Leppa E, Linden AM, Renzi M, Swinny JD et al (2007). From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAA receptors. Nat Neurosci 10: 923–929.
You H, Dunn SM (2007). Identification of a domain in the δ subunit (S238-V264) of the α4β3δ GABAA receptor that confers high agonist sensitivity. J Neurochem 103: 1092–1101.
Acknowledgements
This work was supported by NIH Grants AA10422 (GEH) and DE14184 (DC) and by the Academy of Finland (ERK, AML) and the Sigrid Juselius Foundation (ERK). We thank Bjarke Ebert for a donation of gaboxadol.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chandra, D., Halonen, L., Linden, AM. et al. Prototypic GABAA Receptor Agonist Muscimol Acts Preferentially Through Forebrain High-Affinity Binding Sites. Neuropsychopharmacol 35, 999–1007 (2010). https://doi.org/10.1038/npp.2009.203
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2009.203
Keywords
This article is cited by
-
The role of GPR39 zinc receptor in the modulation of glutamatergic and GABAergic transmission
Pharmacological Reports (2023)
-
The Influence of AA29504 on GABAA Receptor Ligand Binding Properties and Its Implications on Subtype Selectivity
Neurochemical Research (2022)
-
Discovery of a new class of orthosteric antagonists with nanomolar potency at extrasynaptic GABAA receptors
Scientific Reports (2020)
-
Mechanisms of GABAB receptor enhancement of extrasynaptic GABAA receptor currents in cerebellar granule cells
Scientific Reports (2019)
-
Brain allopregnanolone induces marked scratching behaviour in diet-induced atopic dermatitis mouse model
Scientific Reports (2019)