Abstract
The dopamine D2 receptors exist in two states: a high-affinity state (D2high) that is linked to second messenger systems, is responsible for functional effects, and exhibits high affinity for agonists; and a low-affinity state that is functionally inert and exhibits lower affinity for agonists. The dopamine D3 receptors have high-affinity for agonist (eg dopamine) and the existence of the two affinity states is controversial. Although preclinical studies in animal models of psychosis have shown a selective increase of D2high as the common pathway to psychosis, the D3 has been suggested to be involved in the pathophysiology of psychosis. We report the first study of the D2high and D3 in schizophrenia using the novel PET radiotracer, [11C]-(+)-PHNO. We recruited 13 patients with schizophrenia-spectrum disorder amidst an acute psychotic episode, drug free for at least 2 weeks, and 13 age–sex-matched healthy controls. The binding potential no-displaceable (BPND) was examine in the main regions of interest (caudate, putamen, ventral striatum, globus pallidus, substantia nigra, and anterior thalamus) and in a voxel-wise analysis. The BPND between patients and controls was not different in any of the regions. The voxel-wise analysis did not reveal any difference and no correlations were found between the BPND and positive and negative syndrome scale subscales. Our results do not find support for the hypothesis linking psychosis to a selective increase in D2high and/or D3 in schizophrenia. It is possible that receptors with high affinity are not accessible by [11C]-(+)-PHNO because they are occupied by endogenous dopamine, a possibility that can be ruled out in future experiments.
Similar content being viewed by others
INTRODUCTION
It is well established that the dopamine D2/3 receptors are the target of most antipsychotic drugs, though it remains unclear whether the D2/3 receptors are involved in the pathophysiology of psychosis. An important recent development has been the evidence from preclinical models indicating that the ‘high-affinity state’ of the dopamine D2 receptors (D2high) is elevated in psychosis (Seeman et al, 2005b). The dopamine D2 and D3 receptors are members of the G-protein-coupled receptors (GPCR) family. The D2 receptor exists in two inter-convertible states: a G-protein-coupled state which has a high affinity for agonist binding and is responsible for the functional effects of dopamine; and a G-protein-uncoupled state which is a functionally inert state with a low-affinity for dopamine. On the other hand, the existence of the G-protein-coupled and uncoupled states of the D3 receptors is controversial (Freedman et al, 1994; Seeman et al, 2005a; Sokoloff et al, 1992), due to the inability of the G-protein analogs (eg guanilylimidodiphosphate, GppNHp) to shift it from the high- to the low-affinity state (Vanhauwe et al, 2000). Nevertheless, the D3 receptor has at least 20-fold higher affinity than D2 for dopamine (Freedman et al, 1994; Seeman et al, 2005a; Sokoloff et al, 1992).
Data from over a dozen preclinical animal models of psychosis (eg amphetamine sensitization, phencyclidine sensitization, ethanol withdrawal, hippocampal lesion) (Seeman et al, 2005b) suggests that there is a selective elevation of high-affinity states in psychosis. Though, the method of distinguishing high- from low-affinity states in vitro involves the addition of high concentrations of G-protein analogs, a method that is not feasible in humans. Molecular imaging techniques such as positron emission tomography (PET) allow for the in vivo study of brain receptors; but, until recently, the available radioligands for D2/3 receptors (eg [11C]-raclopride, [18F]-fallypride) were all ‘antagonist’ radioligands which would not distinguish between the high- and low-affinity states of the receptors. Studying the high-affinity state of the receptor directly in patients requires the use of an ‘agonist’ radioligand.
Recently, our group has developed [11C]-(+)-PHNO ([11C]-(+)-4-propyl-9-hydroxynaphthoxazine) (Wilson et al, 2005), a D2/3 agonist radiotracer for use in humans. [11C]-(+)-PHNO binds with nanomolar affinity to D2 and D3 receptors allowing for a prominent signal from the striatum, globus pallidus (GP), substantia nigra (SN), and anterior thalamus (Freedman et al, 1994; Graff-Guerrero et al, 2008; Narendran et al, 2006; Seeman et al, 1993; Willeit et al, 2006). It shows at least three critical differences compared to antagonist radiotracers. First, unlike antagonist radiotracers which do not distinguish between the low- and high-affinity states, [11C]-(+)-PHNO is assumed to bind preferentially to the high-affinity states of the receptors (Ginovart et al, 2006; Seeman et al, 2007).
Second, (+)-PHNO has a higher in vitro and in vivo affinity for D3 than for D2 receptors. In vitro, Freedman et al (1994) and Parker et al (2006) reported that (+)-PHNO has more than 10-fold higher affinity for D3 than for D2 using conventional in vitro techniques, though Seeman et al (2005a) reported that (+)-PHNO has a higher affinity for the D2high than D3high) using in vitro analysis in the presence of GppNHp. In vivo, Narendran et al (2006) suggested that [11C]-(+)-PHNO has fourfold higher preference for D3 than D2high—a finding supported by studies in nonhuman primates showing that the [11C]-(+)-PHNO signal in the GP and midbrain binding is selectively displaceable with the D3 preferential agonist (BP897) and antagonist (SB-277011) (Narendran et al, 2006; Rabiner et al, 2007b, 2008). Finally, studies in healthy humans show that the [11C]-(+)-PHNO signal in the GP is preferentially displaceable with the D3 antagonist (ABT925) (Abi-Saab et al, 2008; Graff Guerrero et al, 2008). Thus, across species, methods and techniques, [11C]-(+)-PHNO shows a modest higher affinity for D3 over D2.
Finally, [11C]-(+)-PHNO has shown higher sensitivity for amphetamine displacement than [11C]-raclopride in anesthetized cats (Ginovart et al, 2006). However, this higher sensitivity was not found in an ex vivo dissection study in awake rodents (McCormick et al, 2008), though it is suggested by the study in awake and healthy humans (Willeit et al, 2008). The cause of this discrepancy between studies is still elusive, though the cat and human studies suggest that [11C]-(+)-PHNO binds preferentially to the high-affinity states of the dopamine receptor.
The development of [11C]-(+)-PHNO has finally made it possible to test the hypothesis with regards to elevated high-affinity states in schizophrenia. Moreover, as [11C]-(+)-PHNO provides a robust binding from the D3-rich areas in the brain (GP and SN), for the first time it has provided an opportunity to explore directly the suggested involvement of the dopamine D3 receptors in schizophrenia (Griffon et al, 1995; Gurevich et al, 1997; Joyce and Gurevich, 1999).
The current study was aimed to compare the [11C]-(+)-PHNO binding in drug-free patients with schizophrenia and appropriate controls to test the D2 high-affinity states hypothesis and to compare the dopamine D3 binding between patients with acute psychosis and controls.
MATERIALS AND METHODS
Clinical Sample
This study has been approved by the local Research Ethics Board and by Health Canada. Twenty-six subjects were included: 13 drug-free patients and 13 healthy controls. The participants provided written informed consents after the study procedures and risks were explained. They were excluded if they had a current diagnosis of substance abuse or dependence at screening, positive result in urine drug screen at enrollment or before any of the PET scans, history of clinically significant physical illness, pregnant or lactating women at screening or positive urine pregnancy test before PET scans, or metal implants that would preclude the MRI scan.
The patients were evaluated before PET scan with the clinical global impression (CGI) and positive and negative syndrome scale (PANSS).
The patients had the diagnosis of schizophrenia or schizophreniform disorder corroborated with the MINI-Plus structured interview (Sheehan et al, 1998). The age range for inclusion was from 18 to 50 years old. The patients were drug free for at least 2 weeks and were excluded if they had been exposed to depot antipsychotic in the past 2 years; had a minimum PANSS total score of 55, score of >3 on at least two PANSS psychosis items (P1, P2, P3, P5, or P6) or >4 on one psychosis item; and CGI severity score ⩾4 (moderately ill).
The healthy controls were recruited through local advertisement. Psychiatric disorders were excluded using the MINI-Plus structured interview (Sheehan et al, 1998). The age range for inclusion was from 18 to 50 years old. Subjects with any medical or neurological conditions or with axis I psychiatric diagnoses were excluded from the study. Similarly, subjects with substance abuse (other than smoking) within 6 months before their baseline visit were not included. Participants were asked to consume no more than their usual amount of coffee (and if smokers, cigarettes) on the day of PET examination, and to abstain from alcohol intake 24 h before PET scans. Standard urine tests for psychotropic substances were performed at inclusion and immediately before PET scan. Pregnancy was excluded using serum analysis at inclusion and urine pregnancy tests before each scan.
[11C]-(+)-PHNO Synthesis
The radiosynthesis of [11C]-(+)-PHNO has been described in detail elsewhere (Wilson et al, 2005). Briefly, [11C]-propionyl chloride was reacted with 9-hydroxynaphthoxazine to generate a [11C]-amide which is subsequently reduced by lithium aluminum hydride. Purification by HPLC and formulation gave radiochemically pure [11C]-(+)-PHNO as a sterile, pyrogen-free solution suitable for human studies.
Positron Emission Tomography Imaging
Studies were performed using a high-resolution brain PET camera system, HRRT (Siemens Molecular Imaging, Knoxville, TN), which measures radioactivity in 207 brain slices with an interslice distance of 1.2 mm. The in-plane resolution of the scanner is approximately 2.8 mm full-width-at-half-maximum. Transmission scans were acquired using a 137Cesium single photon point source to provide attenuation correction. The emission data were acquired with a head fixation system during PET scans to avoid movement during the acquisition. After being placed on the scanning table, a total of 329±59 MBq (8.9±1.6 mCi) with a specific activity of 955.9±295 mCi/μmol (range: 407–1790 mCi/μmol) and a mass of 2.33±0.39 μg (range: 1.2–3.3 μg) of [11C]-(+)-PHNO was injected as a bolus followed by a flush with 2 ml saline into an intravenous line placed in an antecubital vein. Scanning data were acquired for 90 min after the injection. Once scanning was completed, the data were reframed into 30 frames (1–15 of 1 min duration and 16–30 of 5 min duration). From the 26 participants in this study, one healthy control experienced transient mild nausea. This was presented few minutes (∼10 min) after [11C]-(+)-PHNO injections and lasted less than 5 min. No interruption of the PET procedures was required due to this adverse effect of the radiotracer.
MRI Imaging
Subjects undertook a proton density image (TE=17, TR=6000, FOV=22 cm 2D, 256 × 256, slice thickness of 2 mm, NEX=2) acquired on a 1.5T Signa scanner (General Electric Medical Systems, Milwaukee, WI). These images were used for the analysis of the PET scans.
Image Analysis
The time activity curves (TACs) from the regions of interest (ROIs) caudate, putamen, ventral striatum (VS), GP, SN, and cerebellar cortex were obtained from the dynamic [11C]-(+)-PHNO PET images in native space with reference to co-registered MRI image as previously described (Graff-Guerrero et al, 2008; Rusjan et al, 2006; Willeit et al, 2008). The ROI in the anterior thalamus was drawn manually using the Analyze Software Version 6.0 (AnalyzeDirect, Lenexa, Kansas) in the anterior part of the thalamus as observed in the first axial projection where putamen and thalamus can be shown. The co-registration of the MRI to the PET space image was carried out using the normalized mutual information algorithm as implemented in SPM2 (SPM2, Welcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm).
The TACs were obtained using a in-house software for semi-automated generation of ROIs (Rusjan et al, 2006). The quantitative estimate of binding was estimated using the simplified reference tissue method (Lammertsma and Hume, 1996) with the cerebellar cortex as reference region. This method has been validated to reliable estimate the binding potential no-displaceable (BPND), which compares the concentration of radioligand in the receptor-rich region to the receptor-free region (Innis et al, 2007), and has been validated for use with [11C]-(+)-PHNO (Ginovart et al, 2007). The BPNDs were estimated using the PMOD v2.7 software (PMOD Technologies Ltd, Zurich, Switzerland).
Parametric voxel-wise BPND maps from the dynamic images in native space were generated according to the method of Gunn et al (1997) with the cerebellum as reference region and as implemented in PMOD v2.7 software (PMOD Technologies Ltd). The BPND map images were spatially normalized into the Montreal Neurological Institute brain space by nearest neighbor interpolation and with a voxel size fixed in 2 × 2 × 2 mm using the SPM2 software (Friston, 1995). The normalized images were smoothed with a Gaussian filter in each coordinate direction with a kernel of 4 mm.
Statistical Analysis
The analysis was made using the Statistical Program for the Social Sciences (version 15.0; SPSS, Chicago, IL). Variables were presented as mean±standard deviation (SD). Demographic and clinical characteristics were compared between patients and controls by using Mann–Whitney U-test (gender) on categorical data and independent sample t-tests on continuous data (age). Analysis of variance with Bonferroni correction for multiple comparisons as a post hoc was performed to compare the BPNDs between drug-free patients and sex- and age-matched healthy controls per ROI (caudate, putamen, VS, GP, SN, and anterior thalamus). Pearson's product moment correlations between PANSS (total and subscales) and BPNDs were estimated. Repeated measures analysis of variance was performed to compare the standardized radioactivity in the cerebellum (within subjects factor) between drug-free patients and sex- and age-matched healthy controls (between subjects factor). The significance was always assumed at p<0.05.
The voxel-wise comparison between BPND maps, of drug-free patients and sex- and age-matched healthy controls, was performed with a two sample t-tests as implemented in SPM2. We reported areas only if they met the joint criteria of (a) p(uncorrected)<0.001; (b) an extent ⩾10 voxels; and (c) in the caudate, putamen, GP, VS, midbrain, and anterior thalamus. Additionally, the statistically significant a priori regions would be corrected for multiple comparisons using the false discovery rate (FDR) approach.
RESULTS
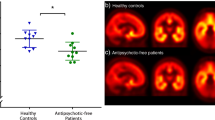
The characteristics of the patients and controls are summarized in Table 1. There were no differences between groups in age (drug-free patients mean=25.8±SD=5.9 years; healthy controls=26.8±6.4 years, t=−0.40, p=0.69) and in gender (drug-free patients=9 men; healthy controls=9 men; Mann–Whitney U-test=84.5, p=1.0). No differences emerged between drug-free patients and age- and sex-matched healthy controls in the BPND in any of the ROI (F(6,19)=0.671, p=0.674). The BPND (mean±SD) in the caudate for controls was 2.1±0.2 and for patients was 2.2±0.4 (p=0.52, pair-wise Bonferroni corrected); putamen: controls=2.6±0.3 and patients=2.6±0.2 (p=0.50); VS: controls=3.1±0.4 and patients=3.2±0.7 (p=0.63); GP: controls=2.9±0.6 and patients=2.7±0.5 (p=0.51); SN: controls=1.5±0.6 and patients=1.3±0.4 (p=0.33); thalamus: controls=1.2±0.2 and patients=1.1±0.3 (p=0.43) (Figure 1). This result was corroborated by the voxel-wise comparison of the BPND maps between drug-free patients and controls which did not show any differences (Figure 2). Moreover, the BPND did not correlate with the severity of the illness as measured by the PANSS even before correction for multiple comparisons (Table 2).
Comparison of the [11C]-(+)-PHNO binding potential no-displaceables (BPNDs) of every region of interests (ROI) between drug-free patients and age- and sex-matched healthy controls. Black symbols indicate drug-naive patients, grey symbols indicate drug-free patients, and white symbols indicate healthy controls. Horizontal lines indicate mean BPND from each ROI. VS, ventral striatum; GP, globus pallidus; SN, substantia nigra.
[11C]-(+)-PHNO mean and standard deviation binding potential no-displaceable (BPND) maps of drug-free patients with schizophrenia (n=13) and age- and sex-matched healthy controls (n=13) illustrating the similitude in binding between controls and patients. The BPND maps images correspond to axial projections overlay in a T1 template in the Montreal Neurological Institute (MNI) space. The images correspond to 13 patients and 13 controls. Z corresponds to the millimeters above (+) or below (−) the anterior commissure in the AC–PC plane.
DISCUSSION
This study represents the first comprehensive investigation of the dopamine D2 receptors in the high-affinity state (D2high) and the D3 receptors in patients. Contrary to our expectation based on preclinical data, we did not find evidence for elevated D2high in untreated patients with schizophrenia or schizophreniform disorder during acute psychosis.
The high-affinity state hypothesis has been sustained in many preclinical models of psychosis (Seeman et al, 2005b) and has been indirectly suggested by dopamine depletion studies in patients (Abi-Dargham et al, 2000). Our results in drug-free patients failed to support this hypothesis. Although the reason for this discrepancy between predictions from in vitro data and findings in patients is not clear, several issues need to be considered.
First, the methods of measuring the high-affinity state in vitro and in patients are very different. The in vitro studies include competition assays between antagonist radiotracers and agonist ligands in the presence of non-hydrolysable GTP analogs (GppNHp), the latter converting the receptor from its high-affinity state to its low-affinity state (De Lean et al, 1980; Seeman et al, 2002, 2005b). This differs from the approach in humans which involves a direct study of the high-affinity states with an agonist radioligand.
Second, there is ongoing debate as to the measurability of high-affinity state in vivo: eg Sibley et al (1983) identified both high- and low-affinity binding sites in membrane cells, but only low-affinity sites on the same intact cells. However, Seeman (2008) identified the high-affinity binding sites on intact cells with [3H]domperidone, but not with [3H]spiperone or [3H]raclopride. Moreover, one PET study in nonhuman primates with [11C]-NPA (D2 agonist) and [11C]-raclopride (D2/3 antagonist) through a Scatchard plot analysis was able to differentiate the density of receptors (Bmax) in the high- vs high+low-affinity state (Narendran et al, 2005); although the same approach in cats with [11C]-(+)-PHNO and [11C]-raclopride did not find any difference in the Bmax between the two radiotracers (Ginovart et al, 2006). On the other hand, studies in anesthetized cats (Ginovart et al, 2006), anesthetized nonhuman primates (Narendran et al, 2004; Seneca et al, 2006), and awake humans (Willeit et al, 2008) showed greater displacement with an agonist radiotracer ([11C]-NPA, [11C]-MNPA, [11C]-(+)-PHNO) than with an antagonist radiotracer in response to an amphetamine challenge. But one ex vivo study in awake rodents did not find any difference in amphetamine displacement between [11C]-(+)-PHNO and [11C]-raclopride (McCormick et al, 2008). These studies suggest that although agonist radiotracers do show a distinct binding profile in vivo, the exact difference varies as a function of the ligand and the tissue/animal characteristics.
Finally, it is theoretically possible that an increase in the D2high and/or D3 is indeed present in acute psychotic state in schizophrenia, but this may be masked by the presence of abnormal high levels of dopamine in the synaptic cleft. The abnormal high levels of dopamine in psychosis has been indirectly shown after an amphetamine challenge (Abi-Dargham et al, 1998), but the dopamine striatal synthesis capacity has reliably been found higher than in controls (Dao-Castellana et al, 1997; Hietala et al, 1999; Huttunen et al, 2007; Reith et al, 1994).
Moreover, if the density of receptors configured in the high-affinity state is extremely high as suggested in normal conditions (∼80%) (Narendran et al, 2005), the difference between patients and controls may be below the resolution of the PET technique. One approach to uncover the differences and to test this hypothesis would require depleting dopamine in patients before imaging studies (Abi-Dargham et al, 2000). We did not deplete dopamine levels and thus cannot definitively rule out a change in D2high/D3 receptors which may have been cancelled by the ambient increase in endogenous dopamine. This is not just a theoretical concern, because studies by Abi-Dargham et al (2000) have shown that patients not only have higher baseline occupancy of dopamine receptors by dopamine, but also that when they are depleted of dopamine, they reveal a higher number of absolute D2 receptors (Abi-Dargham et al, 2000). Thus, a study depleting dopamine would be very critical in definitively resolving this issue.
The ROI and voxel-wise analyses did not reveal any difference in the D3-rich areas (GP and SN) between patients and controls. These results disagree with a previous postmortem study (Gurevich et al, 1997), including drug-free patients with schizophrenia, describing twofold increase in the total number of D3 receptors. The reason of this discrepancy with our results is unclear. But, differences in the sample characteristics between postmortem studies and our data such as age (old vs young), length of illness (years vs months), and previous exposure to antipsychotic medications (Graff-Guerrero et al, 2007) may help to explain the discrepancy on the results.
The current study also provides the first insight to the status of the D3 receptors in the schizophrenia, as the [11C]-(+)-PHNO signal in the SN is predominantly related to D3 (Graff Guerrero et al, 2008; Rabiner et al, 2007a, 2007b). The SN (midbrain) is important because it is the origin of the nigrostriatal dopaminergic system and the D3 receptors in the SN are autoreceptors (Diaz et al, 2000). Thus alterations in these receptors could contribute to the hyperactivity of this midbrain dopamine system which is believed to play an important role in the genesis of schizophrenic symptoms (Murray et al, 2008). However, we do not find support for an alteration of the D3 in the SN.
Another issue to consider is that the [11C]-(+)-PHNO signal reflects its binding to both D2high and D3 receptor populations. The contribution of each receptor to the regional binding varies according to the numbers of each receptor sub-type in the region and to the affinity of the radioligand for that receptor sub-type. Although the precise contribution of D2high and D3 to each ROI is not fully mapped, studies in rodents, baboons, and humans estimate that the vast majority of the [11C]-(+)-PHNO binding in the GP (68–89%) and in the midbrain SN (91–100%) corresponds to D3. On the other hand, the estimation of [11C]-(+)-PHNO binding in the dorsal striatum that corresponds to D3 is between 8 and 50% (Graff Guerrero et al, 2008; Rabiner et al, 2007a, 2007b). These proportions suggest that [11C]-(+)-PHNO binding in the GP and SN is a reasonable proxy for the estimation of D3 receptors in those regions, although the dorsal striatum should be understood as a mixture of predominantly D2high with some contributions from D3. However, this limitation in radioligand selectivity should not take away our empirical finding that we find no difference in the [11C]-(+)-PHNO BPND in drug-free patients with schizophrenia vs controls. Further, our inference that there is no substantial difference in either D2high or D3 is also secure because a change in either of these should have led to an increase in the overall BPND and we do not observe that. There is of course the hypothetical possibility that a simultaneous increase in D2high coupled with a decrease in D3 (or vice versa) could hide a true finding. But, there is no data in the literature that suggest such possibility.
We did not record the smoking status of the healthy controls. This limitation may be important in the light of the effect of cigarette consumption, craving and its mood or hedonic effect on the dopamine system (Barrett et al, 2004; Brody et al, 2008; Montgomery et al, 2007), and the fact that patients with schizophrenia are more likely to be smokers than general population (de Leon and Diaz, 2005). However, given that we found no effect of smoking within our patients with schizophrenia (three smokers vs 10 nonsmokers) (data not shown), we think that it is unlikely that smoking alone could have obscured differences between patients and controls.
The lack of a full kinetic analysis is another potential limitation. This approach would have allowed for the direct estimation of the BP in the ROIs, without the assumption of equivalent nonspecific and free fraction in the reference region. However, our data showed that the cerebellar uptake in the drug-free patients and sex- and age-matched healthy controls was not different (Figure 3; F(1,24)=0.34, p=0.56) and, therefore, it is unlikely that a true finding could have been obscured by differences in the reference region.
Time–activity curves (mean±SD) from the cerebellum of 13 drug-free patients with schizophrenia and 13 age- and sex-matched healthy controls. y axis represents standardized uptake values (SUV; calculated as: regional radioactivity concentration/(injected radioactivity/body weight) for [11C]-(+)-PHNO. The time–activity curves from the cerebellum illustrate that there was no difference between groups on free and nonspecific [11C]-(+)-PHNO binding. Error bars correspond to standard deviation (repeated measure ANOVA, F(1,24)=0.34, p=0.56).
The current study represents the first effort to measure the D2high and D3 receptors in drug-free patients with psychotic disorders (schizophrenia-spectrum disorders). Our results indicate that patients in an acute psychotic episode do not exhibit an increase of the D2high or D3 receptors in comparison to sex- and age-matched healthy controls. We cannot rule out the possibility that any difference of the D2high or D3 were occluded by endogenous dopamine. The next step to definitively rule out such a possibility would require studies using [11C]-(+)-PHNO but in patients whose dopamine has been depleted. Until such a finding is observed, accounts should not assume a change in the D2high or D3 in the pathophysiology of psychosis.
References
Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M et al (1998). Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 155: 761–767.
Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS et al (2000). Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 97: 8104–8109.
Abi-Saab W, Graff-Guerrero A, Redden L, Katz D, Houle S, O'Neill A et al (eds). (2008). First Demonstration of D3 Occupancy in Humans: Blockade of [11C]-(+)-PHNO PET by ABT-925. Society of Biological Psychiatry 2008 Annual Meeting. Washington, DC, USA.
Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A (2004). The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [(11)C]raclopride. Synapse 54: 65–71.
Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL et al (2008). Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology, (2009) 34, –; 18 June 2008; doi:10.1038/npp.2008.87.
Dao-Castellana MH, Paillere-Martinot ML, Hantraye P, Attar-Levy D, Remy P, Crouzel C et al (1997). Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophr Res 23: 167–174.
De Lean A, Stadel JM, Lefkowitz RJ (1980). A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem 255: 7108–7117.
de Leon J, Diaz FJ (2005). A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 76: 135–157.
Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC et al (2000). Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci 20: 8677–8684.
Freedman SB, Patel S, Marwood R, Emms F, Seabrook GR, Knowles MR et al (1994). Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther 268: 417–426.
Friston KJ (1995). Commentary and opinion: II. Statistical parametric mapping: ontology and current issues. J Cereb Blood Flow Metab 15: 361–370.
Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P et al (2006). Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem 97: 1089–1103.
Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S et al (2007). Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab 27: 857–871.
Graff Guerrero A, Abi-Saab W, Redden L, Katz D, Houle S, O'Neill A et al (eds). (2008). First Demonstration of D3 Occupancy in Humans: Blockade of [11C]-(+)-PHNO PET by ABT-925. Collegium Internationale Neuro-Psychopharmacologicum XXVI Congress. Munich, Germany.
Graff-Guerrero A, Mamo D, Agid O, Shammi CM, Mizrahi R, Ginovart N et al (eds). (2007). The D2 and D3 High states in Schizophrenia—Effect of Illness and Antipsychotic Treatment. ACNP 46th Annual Meeting. Boca Raton, Miami, USA.
Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P et al (2008). Brain region binding of the D(2/3) agonist [(11)C]-(+)-PHNO and the D(2/3) antagonist [(11)C]raclopride in healthy humans. Hum Brain Mapp 29: 400–410.
Griffon N, Sokoloff P, Diaz J, Levesque D, Sautel F, Schwartz JC et al (1995). The dopamine D3 receptor and schizophrenia: pharmacological, anatomical and genetic approaches. Eur Neuropsychopharmacol 5 (Suppl): 3–9.
Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ (1997). Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 6: 279–287.
Gurevich EV, Bordelon Y, Shapiro RM, Arnold SE, Gur RE, Joyce JN (1997). Mesolimbic dopamine D3 receptors and use of antipsychotics in patients with schizophrenia. A postmortem study. Arch Gen Psychiatry 54: 225–232.
Hietala J, Syvalahti E, Vilkman H, Vuorio K, Rakkolainen V, Bergman J et al (1999). Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res 35: 41–50.
Huttunen J, Heinimaa M, Svirskis T, Nyman M, Kajander J, Forsback S et al (2007). Striatal dopamine synthesis in first-degree relatives of patients with schizophrenia. Biol Psychiatry 63: 114–117.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539.
Joyce JN, Gurevich EV (1999). D3 receptors and the actions of neuroleptics in the ventral striatopallidal system of schizophrenics. Ann NY Acad Sci 877: 595–613.
Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. Neuroimage 4 (3 Part 1): 153–158.
McCormick PN, Kapur S, Seeman P, Wilson AA (2008). Dopamine D2 receptor radiotracers [(11)C](+)-PHNO and [(3)H]raclopride are indistinguishably inhibited by D2 agonists and antagonists ex vivo. Nucl Med Biol 35: 11–17.
Montgomery AJ, Lingford-Hughes AR, Egerton A, Nutt DJ, Grasby PM (2007). The effect of nicotine on striatal dopamine release in man: A [11C]raclopride PET study. Synapse 61: 637–645.
Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G et al (2008). Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry 13: 239, 267–276.
Narendran R, Hwang DR, Slifstein M, Hwang Y, Huang Y, Ekelund J et al (2005). Measurement of the proportion of D2 receptors configured in state of high affinity for agonists in vivo: a Positron Emission Tomography study using [11C]N-propyl-norapomorphine and [11C]raclopride in baboons. J Pharmacol Exp Ther 315: 80–90.
Narendran R, Hwang DR, Slifstein M, Talbot PS, Erritzoe D, Huang Y et al (2004). In vivo vulnerability to competition by endogenous dopamine: Comparison of the D2 receptor agonist radiotracer (−)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse 52: 188–208.
Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E et al (2006). Dopamine (D(2/3)) receptor agonist positron emission tomography radiotracer [(11)C]-(+)-PHNO is a D(3) receptor preferring agonist in vivo. Synapse 60: 485–495.
Parker C, Clarke K, Gee AD, Gee A, Rabiner E (2006). In vitro characterisation of the high affinity D2/D3 dopamine agonist (+)PhNO. NeuroImage 31 (Suppl 2): T29.
Rabiner E, Raymond R, Diwan M, McCormick P, Wilson A, Nobrega J (eds). (2007a). D3 and D2 components of ex vivo regional (+)-PHNO brain binding in wild-type and knock-out mice. Society of Nuclear Medicine 2007 Annual Meeting. Washington, DC, USA.
Rabiner E, Slifstein M, Gunn K, Gentile G, Cunningham VJ, Narendran R et al (eds). (2007b). D3 Receptor Quantification with [11C]-(+)-PHNO PET. ACNP 46th Annual Meeting. Boca Raton, Miami, USA.
Rabiner E, Slifstein M, Gunn R, Gentile G, Cunningham VJ, Xu Y . et al (eds). (2008). First Quantification of D3 Receptors in vivo: PET with [11C]PHNO and [11C]Fallypride.. Society of Biological Psychiatry 2008 Annual Meeting. Whashington, DC, USA.
Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F et al (1994). Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci USA 91: 11651–11654.
Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F et al (2006). An automated method for the extraction of regional data from PET images. Psychiatry Res 147: 79–89.
Seeman P (2008). Dopamine D2(high) receptors on intact cells. Synapse 62: 314–318.
Seeman P, Ko F, Willeit M, McCormick P, Ginovart N (2005a). Antiparkinson concentrations of pramipexole and PHNO occupy dopamine D2(high) and D3(high) receptors. Synapse 58: 122–128.
Seeman P, McCormick PN, Kapur S (2007). Increased dopamine D2(high) receptors in amphetamine-sensitized rats, measured by the agonist [(3)H](+)PHNO. Synapse 61: 263–267.
Seeman P, Tallerico T, Ko F, Tenn C, Kapur S (2002). Amphetamine-sensitized animals show a marked increase in dopamine D2 high receptors occupied by endogenous dopamine, even in the absence of acute challenges. Synapse 46: 235–239.
Seeman P, Ulpian C, Larsen RD, Anderson PS (1993). Dopamine receptors labelled by PHNO. Synapse 14: 254–262.
Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK et al (2005b). Dopamine supersensitivity correlates with D2high states, implying many paths to psychosis. Proc Natl Acad Sci USA 102: 3513–3518.
Seneca N, Finnema SJ, Farde L, Gulyas B, Wikstrom HV, Halldin C et al (2006). Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: a comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse 59: 260–269.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 (Suppl 20): 22–33; quiz 34–57.
Sibley DR, Mahan LC, Creese I (1983). Dopamine receptor binding on intact cells. Absence of a high-affinity agonist-receptor binding state. Mol Pharmacol 23: 295.
Sokoloff P, Andrieux M, Besancon R, Pilon C, Martres MP, Giros B et al (1992). Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur J Pharmacol 225: 331–337.
Vanhauwe JF, Josson K, Luyten WH, Driessen AJ, Leysen JE (2000). G-protein sensitivity of ligand binding to human dopamine D(2) and D(3) receptors expressed in Escherichia coli: clues for a constrained D(3) receptor structure. J Pharmacol Exp Ther 295: 274–283.
Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D et al (2005). Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem 48: 4153–4160.
Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S et al (2008). First human evidence of d-amphetamine induced displacement of a D(2/3) agonist radioligand: a [(11)C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology 33: 279–289.
Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P et al (2006). High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol Psychiatry 59: 389–394.
Acknowledgements
The authors thank Armando Garcia, Winston Stableford, Min Wong, Alvina Ng, Terry Bell, Ted Harris-Brandts, and Peter Bloomfield for their technical assistance. This work was supported by a grant from the Canadian Institutes for Health Research. Funding of the PET camera system HRRT was supported by the Canada Foundation for Innovation, the Ontario Innovation Trust, and the Ontario Research and Development Challenge Fund. The study was funded by Operating Grant no. 157739 by the Canadian Institutes of Health Research to SK. AG-G is partially supported by SNI-CONACyT. The data relating to drug-free patients have been reported by RM as part of her PhD thesis submitted to the University of Toronto in 2007.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICTS OF INTEREST
Dr Graff-Guerrero has received professional services compensation from Abbott Laboratories and grant support from Janssen. Dr Mizrahi reports no competing interests. Dr Agid reports no competing interests. BSc Marcon reports no competing interests. RN Barsoum reports no competing interests. Dr Rusjan reports no competing interests. Dr Wilson reports no competing interests. Dr Zipursky reports receiving grant support from Eli Lilly as well as serving as a consultant, scientific advisor, or speaker for AstraZeneca, Eli Lilly and Novartis. Dr Kapur has received grant support from or has served as a consultant, scientific advisor, or speaker for AstraZeneca, Bristol-Meyers Squibb, Eli Lilly, EMD, Darmstadt, GlaxoSmithKline, Janssen, Neuromolecular, Otsuka, Organon, Pfizer, Sanofi-Synthelabo, Servier, Solvay/Wyeth and Abbott.
Rights and permissions
About this article
Cite this article
Graff-Guerrero, A., Mizrahi, R., Agid, O. et al. The Dopamine D2 Receptors in High-Affinity State and D3 Receptors in Schizophrenia: A Clinical [11C]-(+)-PHNO PET Study. Neuropsychopharmacol 34, 1078–1086 (2009). https://doi.org/10.1038/npp.2008.199
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2008.199
Keywords
This article is cited by
-
Thalamic dopamine D2-receptor availability in schizophrenia: a study on antipsychotic-naive patients with first-episode psychosis and a meta-analysis
Molecular Psychiatry (2022)
-
Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [11C]-(+)-PHNO
Psychopharmacology (2016)
-
The neurobiology and treatment of first-episode schizophrenia
Molecular Psychiatry (2015)
-
In Vivo Imaging of Cerebral Dopamine D3 Receptors in Alcoholism
Neuropsychopharmacology (2014)
-
Direct and indirect interactions of the dopamine D3 receptor with glutamate pathways: implications for the treatment of schizophrenia
Naunyn-Schmiedeberg's Archives of Pharmacology (2013)