Abstract
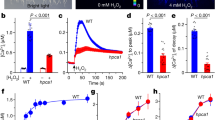
Plants need to cope with biotic and abiotic stress through well-coordinated cell-to-cell communication to survive as sedentary organisms. Environmental challenges such as wounding, low temperature, oxidative states and pathogen infection are known to affect the symplasmic molecular exchange between plant cells determined by plasmodesmal permeability. However, the signalling pathways and mechanisms by which different environmental stressors affect plasmodesmal permeability are not well understood. Here we show that regulating callose accumulation at plasmodesmal channels is a common strategy to alter plasmodesmal permeability under both pathogen infection and mechanical wounding stress. We have identified Arabidopsis callose synthase 1 (CalS1) and CalS8 as key genes involved in this process, and have integrated these new players into both known and novel signalling pathways that control responses to biotic and abiotic stress. Our studies provide experimental data that indicate the presence of specialized pathways tuned to respond to particular stressors, and new insights into how plants regulate plasmodesmata in response to environmental assaults.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Abounit, S. & Zurzolo, C. Wiring through tunneling nanotubes—from electrical signals to organelle transfer. J. Cell Sci. 125, 1089–1098 (2012).
Gerdes, H. H., Rustom, A. & Wang, X. Tunneling nanotubes, an emerging intercellular communication route in development. Mech. Develop. 130, 381–387 (2013).
Lee, J. Y. New and old roles of plasmodesmata in immunity and parallels to tunneling nanotubes. Plant Sci. 221–222, 13–20 (2014).
Robards, A. W. & Lucas, W. J. Plasmodesmata. Annu. Rev. Plant Phys. 41, 369–419 (1990).
Oparka, K. J. Getting the message across: how do plant cells exchange macromolecular complexes? Trends Plant Sci. 9, 33–41 (2004).
Lucas, W. J. & Lee, J. Y. Plasmodesmata as a supracellular control network in plants. Nature Rev. Mol. Cell Biol. 5, 712–726 (2004).
Lucas, W. J., Ham, B. K. & Kim, J. Y. Plasmodesmata—bridging the gap between neighboring plant cells. Trends Cell Biol. 19, 495–503 (2009).
Benitez-Alfonso, Y., Jackson, D. & Maule, A. Redox regulation of intercellular transport. Protoplasma 248, 131–140 (2011).
Wu, S. & Gallagher, K. L. Transcription factors on the move. Curr. Opin. Plant Biol. 15, 645–651 (2012).
Burch-Smith, T. M. & Zambryski, P. C. Plasmodesmata paradigm shift: regulation from without versus within. Annu. Rev. Plant Biol. 63, 239–260 (2012).
Sevilem, I., Miyashima, S. & Helariutta, Y. Cell-to-cell communication via plasmodesmata in vascular plants. Cell Adh. Migr. 7, 27–32 (2013).
Sager, R. & Lee, J.-Y. Plasmodesmata in integrated cell signaling: new insights from development and environmental signals and stresses. J. Exp. Bot. 65, 6337–6358 (2014).
Ehlers, K. & Westerloh, M. G. in Symplasmic Transport in Vascular Plants (eds Sokolowska, K. & Sowinski, P. ) 41–82 (Springer Science, 2013).
Zavaliev, R., Ueki, S., Epel, B. L. & Citovsky, V. Biology of callose (beta-1,3-glucan) turnover at plasmodesmata. Protoplasma 248, 117–130 (2011).
De Storme, N. & Geelen, D. Callose homeostasis at plasmodesmata: molecular regulators and developmental relevance. Front. Plant Sci. 5, 138 (2014).
Iglesias, V. A. & Meins, F. Jr . Movement of plant viruses is delayed in a beta-1,3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 21, 157–166 (2000).
Levy, A., Erlanger, M., Rosenthal, M. & Epel, B. L. A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. Plant J. 49, 669–682 (2007).
Simpson, C., Thomas, C., Findlay, K., Bayer, E. & Maule, A. J. An Arabidopsis GPI-anchor plasmodesmata neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell 21, 581–594 (2009).
Lee, J. Y. & Lu, H. Plasmodesmata: the battleground against intruders. Trends Plant Sci. 16, 201–210 (2011).
Guseman, J. M. et al. Dysregulation of cell-to-cell connectivity and stomatal patterning by loss-of-function mutation in Arabidopsis chorus (glucan synthase-like 8). Development 137, 1731–1741 (2010).
Vaten, A. et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 21, 1144–1155 (2011).
Benitez-Alfonso, Y. et al. Symplastic intercellular connectivity regulates lateral root patterning. Dev. Cell 26, 136–147 (2013).
Han, X. et al. Auxin-callose-mediated plasmodesmata gating is essential for tropic auxin gradient formation and signaling. Dev. Cell 28, 132–146 (2014).
Lee, J. Y. et al. A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell 23, 3353–3373 (2011).
Wang, X. et al. Salicylic acid regulates plasmodesmata closure during innate immune responses in Arabidopsis. Plant Cell 25, 2315–2329 (2013).
Cui, W., Wang, X. & Lee, J. Y. Drop-ANd-See: a simple, real-time, and noninvasive technique for assaying plasmodesmata permeability. Methods Mol. Biol. 1217, 149–156 (2015).
Rao, M. V., Paliyath, G., Ormrod, D. P., Murr, D. P. & Watkins, C. B. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Salicylic acid-mediated oxidative damage requires H2O2 . Plant Physiol. 115, 137–149 (1997).
Leon, J., Lawton, M. A. & Raskin, I. Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiol. 108, 1673–1678 (1995).
Shirasu, K., Nakajima, H., Rajasekhar, V. K., Dixon, R. A. & Lamb, C. Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell 9, 261–270 (1997).
Torres, M. A., Jones, J. D. & Dangl, J. L. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nature Genet. 37, 1130–1134 (2005).
Straus, M. R., Rietz, S., van Themaat, E. V. L., Bartsch, M. & Parker, J. E. Salicylic acid antagonism of EDS1-driven cell death is important for immune and oxidative stress responses in Arabidopsis. Plant J. 62, 628–640 (2010).
Fu, Z. Q. & Dong, X. Systemic acquired resistance: turning local infection into global defense. Annu. Rev. Plant Biol. 64, 839–863 (2013).
Radford, J. E., Vesk, M. & Overall, R. L. Callose deposition at plasmodesmata. Protoplasma 201, 30–37 (1998).
Orozco-Cardenas, M. & Ryan, C. A. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc. Natl Acad. Sci. USA 96, 6553–6557 (1999).
Miller, G. et al. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal 2, ra45 (2009).
Suzuki, N. et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25, 3553–3569 (2013).
Pogany, M. et al. Dual roles of reactive oxygen species and NADPH oxidase RBOHD in an Arabidopsis-Alternaria pathosystem. Plant Physiol. 151, 1459–1475 (2009).
Hieno, A. et al. Analysis of environmental stress in plants with the aid of marker genes for H2O2 responses. Methods Enzymol. 527, 221–237 (2013).
Hong, Z., Delauney, A. J. & Verma, D. P. A cell plate-specific callose synthase and its interaction with phragmoplastin. Plant Cell 13, 755–768 (2001).
Acknowledgements
We thank K. Gallagher for her critical comments on the manuscript and editing, and S. Xiao and P. Kachroo for helpful discussions. We also thank Lee laboratory members and J. Caplan for technical assistance and J. Yoo for editing and proofreading the manuscript. This work was supported partially through the grants provided by the National Science Foundation (IOS-0954931 and IOS-1457121) and through grants from the National Center for Research Resources (5P30RR031160-03) and IDeA program of the National Institute of General Medical Sciences (P30 GM103519), which are awarded to J.-Y.L.
Author information
Authors and Affiliations
Contributions
W.C. performed the experiments and J.-Y.L. designed the experiments. W.C. and J.-Y.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Cui, W., Lee, JY. Arabidopsis callose synthases CalS1/8 regulate plasmodesmal permeability during stress. Nature Plants 2, 16034 (2016). https://doi.org/10.1038/nplants.2016.34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.34
This article is cited by
-
Large-scale identification of genes involved in septal pore plugging in multicellular fungi
Nature Communications (2023)
-
The maize callose synthase SLM1 is critical for a normal growth by controlling the vascular development
Molecular Breeding (2023)
-
The receptor kinase SRF3 coordinates iron-level and flagellin dependent defense and growth responses in plants
Nature Communications (2022)
-
Physiological and molecular advances in magnesium nutrition of plants
Plant and Soil (2021)