Abstract
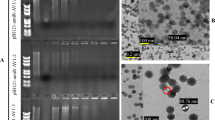
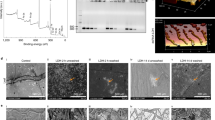
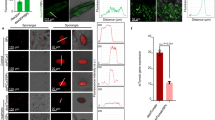
Topical application of pathogen-specific double-stranded RNA (dsRNA) for virus resistance in plants represents an attractive alternative to transgenic RNA interference (RNAi). However, the instability of naked dsRNA sprayed on plants has been a major challenge towards its practical application. We demonstrate that dsRNA can be loaded on designer, non-toxic, degradable, layered double hydroxide (LDH) clay nanosheets. Once loaded on LDH, the dsRNA does not wash off, shows sustained release and can be detected on sprayed leaves even 30 days after application. We provide evidence for the degradation of LDH, dsRNA uptake in plant cells and silencing of homologous RNA on topical application. Significantly, a single spray of dsRNA loaded on LDH (BioClay) afforded virus protection for at least 20 days when challenged on sprayed and newly emerged unsprayed leaves. This innovation translates nanotechnology developed for delivery of RNAi for human therapeutics to use in crop protection as an environmentally sustainable and easy to adopt topical spray.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Flood, J. The importance of plant health to food security. Food Secur. 2, 215–231 (2010).
Bebber, D. P., Ramotowski, M. A. T. & Gurr, S. J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Change 3, 985–988 (2013).
Baulcombe, D. RNA silencing in plants. Nature 431, 356–363 (2004).
Bartel, D. P. MicroRNAs: genomics, biogensis, mechanism and function. Cell 116, 281–297 (2004).
Burand, J. P. & Hunter, W. B. RNAi: future in insect management. J. Invertebr. Pathol. 112, S68–S74 (2013).
Mailard, P. V. et al. Antiviral RNA interference in mammalian cells. Science 342, 235–238 (2013).
Borges, F. & Martienssen, R. A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 16, 727–741 (2015).
Gordon, K. H. J. & Waterhouse, P. M. RNAi for insect-proof plants. Nat. Biotechnol. 25, 1231–1232 (2007).
Lilley, C. J., Davies, L. J. & Urwin, P. E. RNA interference in plant parasitic nematodes: a summary of the current status. Parasitology 139, 630–640 (2012).
Koch, A. et al. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase–encoding genes confers strong resistance to Fusarium species. Proc. Natl Acad. Sci. USA 110, 19324–19329 (2013).
Duan, C.-G., Wang, C.-H. & Guo, H.-S. Application of RNA silencing to plant disease resistance. Silence 3, 5 (2012).
Robinson, K. E., Worrall, E. A. & Mitter, N. Double stranded RNA expression and its topical application for non-transgenic resistance to plant viruses. J. Plant Biochem. Biotechnol. 23, 231–237 (2014).
Tenllado, F., Llave, C. & Diaz-Ruiz, J. R. RNA interference as a new biotechnological tool for the control of virus diseases in plants. Virus Res. 102, 85–96 (2004).
Tenllado, F. & Díaz-Ruíz, J. R. Double-stranded RNA-mediated interference with plant virus infection. J. Virol. 75, 12288–12297 (2001).
Tenllado, F., Martínez-García, B., Vargas, M. & Díaz-Ruíz, J. R. Crude extracts of bacterially expressed dsRNA can be used to protect plants against virus infections. BMC Biotechnol. 3, 3 (2003).
Gan, D. et al. Bacterially expressed dsRNA protects maize against SCMV infection. Plant Cell Rep. 29, 1261–1268 (2010).
Lau, S. E. et al. Crude extracts of bacterially-expressed dsRNA protect orchid plants against Cymbidium mosaic virus during transplantation from in vitro culture. J. Hortic. Sci. Biotechnol. 89, 569–576 (2014).
Xu, Z. P. et al. Stable suspension of layered double hydroxide nanoparticles in aqueous solution. J. Am. Chem. Soc. 128, 36–37 (2006).
Ram Reddy, M. K., Xu, Z. P., Lu, G. Q. & Diniz da Costa, J. C. Layered double hydroxides for CO2 capture: structure evolution and regeneration. Ind. Eng. Chem. Res. 45, 7504–7509 (2006).
Ram Reddy, M. K., Xu, Z. P., Lu, G. Q. (Max) & Diniz da Costa, J. C. Effect of SOx adsorption on layered double hydroxides for CO2 capture. Ind. Eng. Chem. Res. 47, 7357–7360 (2008).
Xu, Z. & Zeng, H. Abrupt structural transformation in hydrotalcite-like compounds Mg1-xAlx(OH)2(NO3)x.nH2O as a continuous function of nitrate anions. J. Phys. Chem. B 105, 1743–1749 (2001).
Dong, H. et al. Engineering small MgAl-layered double hydroxide nanoparticles for enhanced gene delivery. Appl. Clay Sci. 100, 66–75 (2014).
Cavani, F., Trifirò, F. & Vaccari, A. Hydrotalcite-type anionic clays: preparation, properties and applications. Catalysis Today 11, 173–301 (1991).
Dietzgen, R. & Mitter, N. Transgenic gene silencing strategies for virus control. Australas. Plant Pathol. 35, 605–618 (2006).
Li, H.-W. et al. Strong host resistance targeted against a viral suppressor of the plant gene silencing defence mechanism. EMBO J. 18, 2683–2691 (1999).
Ladewig, K., Niebert, M., Xu, Z. P., Gray, P. P. & Lu, G. Q. M. Efficient siRNA delivery to mammalian cells using layered double hydroxide nanoparticles. Biomaterials 31, 1821–1829 (2010).
Elmayan, T. et al. Arabidopsis mutants impaired in cosuppression. Plant Cell 10, 1747–1757 (1998).
Roossinck, M. J. Evolutionary history of Cucumber mosaic virus deduced by phylogenetic analyses. J. Virol. 76, 3382–3387 (2002).
Kanasty, R., Dorkin, J. R., Vegas, A. & Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 12, 967–977 (2013).
Wittrup, A. et al. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 33, 870–876 (2015).
Jiang, L. et al. Systemic gene silencing in plants triggered by fluorescent nanoparticle-delivered double-stranded RNA. Nanoscale 6, 9965–9969 (2014).
Ladewig, K., Xu, Z. P. & Lu, G. Q. (Max). Layered double hydroxide nanoparticles in gene and drug delivery. Expert Opin. Drug Deliv. 6, 907–922 (2009).
Li, H., Guan, R., Guo, H. & Miao, X. New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant Cell Environ. 38, 2277–2285 (2015).
Lau, S.-E., Schwarzacher, T., Othman, R. Y. & Harikrishna, J. A. dsRNA silencing of an R2R3-MYB transcription factor affects flower cell shape in a Dendrobium hybrid. BMC Plant Biol. 15, 194 (2015).
Molnar, A. et al. Plant virus-derived small interfering RNAs originate predominantly from high structured single-strand viral RNAs. J. Virol. 79, 7812–7818 (2005).
Donaire, L. et al. Deep-sequencing of plant viral small RNAs reveals effective and widespread targeting of viral genomes. Virology 392, 203–214 (2009).
Mitter, N., Koundal, V., Williams, S. & Pappu, H. Differential expression of tomato spotted wilt virus-derived viral small RNAs in infected commercial and experimental host plants. PLoS ONE 8, e76276 (2013).
Mitter, N. & Dietzgen, R. G. Use of hairpin RNA constructs for engineering plant virus resistance. Methods Mol. Biol. 894, 191–208 (2012).
Mitter, N., Sulistyowati, E. & Dietzgen, R. G. Cucumber mosaic virus infection transiently breaks dsRNA-induced transgenic immunity to Potato virus Y in tobacco. Am. Phytopathological Soc. 16, 936–944 (2003).
Sulistyowati, E., Mitter, N., Bastiaan-Net, S., Roossinck, M. J. & Dietzgen, R. G. Host range, symptom expression and RNA 3 sequence analyses of six Australian strains of Cucumber mosaic virus. Australas. Plant Pathol. 33, 505–512 (2004).
Acknowledgements
This work was supported by the Bill and Melinda Gates Foundation Grand Challenges Exploration Grant and the University of Queensland's Collaborative Industry Engagement Fund followed by Accelerated Partnership Grant, Queensland Government awarded to N.M. and the ARC Future Fellowship (FT120100813) awarded to Z.P.X. Special thanks goes to M. Pointon and B. Duggan from Nufarm Australia Ltd as the industry partner and D. Ferguson from Uniquest, the commercialization arm of the University of Queensland for support and suggestions. We thank K. Vinall for technical assistance with the confocal microscopy studies. The PMMoVIR54 construct was a kind gift provided by F. Tenllado, Centro de Investigaciones Biológicas, Madrid, Spain. E.A.W. PhD programme with N.M. is supported by a scholarship from the University of Queensland.
Author information
Authors and Affiliations
Contributions
N.M., Z.P.X. and G.Q.L. conceived the BioClay technology. N.M., E.A.W., K.E.R., B.J.C. and Z.P.X. wrote the manuscript. N.M., Z.P.X. and B.J.C. provided expertise and supervised the work. E.A.W. preformed the experiments on constructs and expression of dsRNA, dsRNA loading into LDH, breakdown of LDH and release of dsRNA, stability of dsRNA bound to LDH, dsRNA uptake studies and crop protection assays. K.E.R. preformed experiments on constructs and expression of dsRNA, dsRNA loading into LDH, stability of dsRNA bound to LDH and crop protection assays. P.L. preformed experiments on LDH synthesis and characterization. R.G.J. preformed experiments on the northern blot detection of dsRNA uptake. C.T. preformed experiments on the GUS reporter system. S.J.F. preformed all statistical and bioinformatics analyses. All authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–11, Supplementary Tables 1 and 2. (PDF 1674 kb)
Rights and permissions
About this article
Cite this article
Mitter, N., Worrall, E., Robinson, K. et al. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nature Plants 3, 16207 (2017). https://doi.org/10.1038/nplants.2016.207
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.207
This article is cited by
-
Synthesis of eco-friendly layered double hydroxide and nanoemulsion for jasmine and peppermint oils and their larvicidal activities against Culex pipiens Linnaeus
Scientific Reports (2024)
-
Kaolinite nanoclay-shielded dsRNA drenching for management of Globodera pallida: An environmentally friendly pest management approach
Protoplasma (2024)
-
Nanoparticle-mediated dsRNA delivery for precision insect pest control: a comprehensive review
Molecular Biology Reports (2024)
-
Advances in RNA Interference for Plant Functional Genomics: Unveiling Traits, Mechanisms, and Future Directions
Applied Biochemistry and Biotechnology (2024)
-
Can biocontrol be the game-changer in integrated pest management? A review of definitions, methods and strategies
Journal of Plant Diseases and Protection (2024)