Abstract
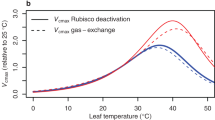
Enhancing the catalytic properties of the CO2-fixing enzyme Rubisco is a target for improving agricultural crop productivity. Here, we reveal extensive diversity in the kinetic response between 10 and 37 °C by Rubisco from C3 and C4 species within the grass tribe Paniceae. The CO2 fixation rate () for Rubisco from the C4 grasses with nicotinamide adenine dinucleotide (NAD) phosphate malic enzyme (NADP-ME) and phosphoenolpyruvate carboxykinase (PCK) photosynthetic pathways was twofold greater than the of Rubisco from NAD-ME species across all temperatures. The declining response of CO2/O2 specificity with increasing temperature was less pronounced for PCK and NADP-ME Rubisco, which would be advantageous in warmer climates relative to the NAD-ME grasses. Modelled variation in the temperature kinetics of Paniceae C3 Rubisco and PCK Rubisco differentially stimulated C3 photosynthesis relative to tobacco above and below 25 °C under current and elevated CO2. Amino acid substitutions in the large subunit that could account for the catalytic variation among Paniceae Rubisco are identified; however, incompatibilities with Paniceae Rubisco biogenesis in tobacco hindered their mutagenic testing by chloroplast transformation. Circumventing these bioengineering limitations is critical to tailoring the properties of crop Rubisco to suit future climates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ort, D. R. et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl Acad. Sci. USA 112, 8529–8536 (2015).
Zhu, X.-G., Long, S. P. & Ort, D. R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61, 235–261 (2010).
Long, S. P., Marshall-Colon, A. & Zhu, X.-G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66 (2015).
Carmo-Silva, E., Scales, J. C., Madgwick, P. J. & Parry, M. A. J. Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Env. 38, 1817–1832 (2015).
Evans, J. R. Improving photosynthesis. Plant Physiol. 162, 1780–1793 (2013).
Parry, M. A. J. et al. Rubisco activity and regulation as targets for crop improvement. J. Exp. Bot. 64, 717–730 (2013).
von Caemmerer, S., Quick, W. P. & Furbank, R. T. The development of C4 rice: current progress and future challenges. Science 336, 1671–1672 (2012).
Whitney, S. M., Houtz, R. L. & Alonso, H. Advancing our understanding and capacity to engineer nature's CO2-sequestering enzyme, Rubisco. Plant Physiol. 155, 27–35 (2011).
Andersson, I. Catalysis and regulation in Rubisco. J. Exp. Bot. 59, 1555–1568 (2008).
Andersson, I. & Backlund, A. Structure and function of Rubisco. Plant Physiol. Biochem. 46, 275–291 (2008).
Sharwood, R. E., Ghannoum, O. & Whitney, S. M. Prospects for improving CO2 fixation in C3-crops through understanding C4-Rubisco biogenesis and catalytic diversity. Curr. Opin. Plant Biol. 31, 135–142 (2016).
Carmo-Silva, A. E. et al. Rubisco activities, properties, and regulation in three different C4 grasses under drought. J. Exp. Bot. 61, 2355–2366 (2010).
Galmés, J. et al. Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends. Plant Cell Environ. 37, 1989–2001 (2014).
Galmés, J., Kapralov, M. V., Copolovici, L. O., Hermida-Carrera, C. & Niinemets, Ü. Temperature responses of the Rubisco maximum carboxylase activity across domains of life: phylogenetic signals, trade-offs, and importance for carbon gain. Photosynth. Res. 123, 183–201 (2015).
Jordan, D. B. & Ogren, W. L. The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase oxygenase: dependence on ribulosebisphosphate concentration, pH and temperature. Planta. 161, 308–313 (1984).
Prins, A. et al. Rubisco catalytic properties of wild and domesticated relatives provide scope for improving wheat photosynthesis. J. Exp. Bot. 67, 1827–1838 (2016).
Young, J. N. et al. Large variation in the Rubisco kinetics of diatoms reveals diversity among their carbon-concentrating mechanisms. J. Exp. Bot. 67, 3445–3456 (2016).
Andrews, T. J. & Whitney, S. M. Manipulating ribulose bisphosphate carboxylase/oxygenase in the chloroplasts of higher plants. Arch. Biochem. Biophys. 414, 159–169 (2003).
Raven, J. A. Rubisco: still the most abundant protein of Earth? New Phytol. 198, 1–3 (2013).
Sage, R. F. The evolution of C4 photosynthesis. New Phytol. 161, 341–370 (2004).
Sage, R. F., Christin, P.-A. & Edwards, E. J. The C4 plant lineages of planet Earth. J. Exp. Bot. 62, 3155–3169 (2011).
Furbank, R. T. Evolution of the C4 photosynthetic mechanism: are there really three C4 acid decarboxylation types? J. Exp. Bot. 62, 3103–3108 (2011).
Sage, R. F., Sage, T. L. & Kocacinar, F. Photorespiration and the evolution of C4 photosynthesis. Annu. Rev. Plant Biol. 63, 19–47 (2012).
Ghannoum, O. et al. Faster rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiol. 137, 638–650 (2005).
Tcherkez, G. G. B., Farquhar, G. D. & Andrews, T. J. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl Acad. Sci. USA 103, 7246–7251 (2006).
Boyd, R. A., Gandin, A. & Cousins, A. B. Temperature response of C4 photosynthesis: biochemical analysis of Rubisco, phosphoenolpyruvate carboxylase and carbonic anhydrase in Setaria viridis. Plant Physiol. 169, 1850–1861 (2015).
Perdomo, J. A., Cavanagh, A. P., Kubien, D. S. & Galmés, J. Temperature dependence of in vitro Rubisco kinetics in species of Flaveria with different photosynthetic mechanisms. Photosynth. Res. 124, 67–75 (2015).
Sage, R. F. Variation in the k cat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. J. Exp. Bot. 53, 609–620 (2002).
Savir, Y., Noor, E., Milo, R. & Tlusty, T. Cross-species analysis traces adaptation of Rubisco toward optimality in a low-dimensional landscape. Proc. Natl Acad. Sci. USA 107, 3475–3480 (2010).
Pearcy, R. W. & Ehleringer, J. Comparative ecophysiology of C3 and C4 plants. Plant Cell Environ. 7, 1–13 (1984).
Tcherkez, G. The mechanism of Rubisco-catalyzed oxygenation. Plant Cell Environ. 39, 983–997 (2016).
Farquhar, G. D., von Caemmerer, S. & Berry, J. A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 (1980).
Sharwood, R. E. & Whitney, S. M. Correlating Rubisco catalytic and sequence diversity within C3 plants with changes in atmospheric CO2 concentrations. Plant Cell Environ. 37, 1981–1984 (2014).
Sharkey, T. D., Bernacchi, C. J., Farquhar, G. D. & Singsaas, E. L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 30, 1035–1040 (2007).
Walker, B., Ariza, L. S., Kaines, S., Badger, M. R. & Cousins, A. B. Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum. Plant Cell Environ. 36, 2108–2119 (2013).
Lauteri, M., Haworth, M., Serraj, R., Monteverdi, M. C. & Centritto, M. Photosynthetic diffusional constraints affect yield in drought stressed rice cultivars during flowering. PLoS ONE 9, e109054 (2014).
Olsovska, K. et al. Genotypically identifying wheat mesophyll conductance regulation under progressive drought stress. Front. Plant Sci. 7, 1111 (2016).
Hermida-Carrera, C., Kapralov, M. V. & Galmés, J. Rubisco catalytic properties and temperature response in crops. Plant Physiol. 171, 2549–2561 (2016).
Orr, D. et al. Surveying Rubisco diversity and temperature response to improve crop photosynthetic efficiency. Plant Physiol. 172, 707–717 (2016).
Badger, M. R. & Collatz, G. J. Studies on the kinetic mechanism of RuBP carboxylase and oxygenase reactions, with particular reference to the effect of temperature on kinetic parameters. Carnegie YB 76, 355–361 (1977).
Sharwood, R. E., Sonawane, B. V., Ghannoum, O. & Whitney, S. M. Improved analysis of C4 and C3 photosynthesis via refined in vitro assays of their carbon fixation biochemistry. J. Exp. Bot. 67, 3137–3148 (2016).
von Caemmerer, S. & Furbank, R. T. The C4 pathway: an efficient CO2 pump. Photosynth. Res. 77, 191–207 (2003).
Still, C. J., Pau, S. & Edwards, E. J. Land surface skin temperature captures thermal environments of C3 and C4 grasses. Glob. Ecol. Biogeo. 23, 286–296 (2014).
Galmés, J. et al. Environmentally driven evolution of Rubisco and improved photosynthesis and growth within the C3 genus Limonium (Plumbaginaceae). New Phytol. 203, 989–999 (2014).
Whitney, S. M. & Andrews, T. J. Plastome-encoded bacterial ribulose-1, 5-bisphosphate carboxylase/oxygenase (RubisCO) supports photosynthesis and growth in tobacco. Proc. Natl Acad. Sci. USA 98, 14738–14743 (2001).
Ishikawa, C., Hatanaka, T., Misoo, S., Miyake, C. & Fukayama, H. Functional incorporation of sorghum small subunit increases the catalytic turnover rate of Rubisco in transgenic rice. Plant Physiol. 156, 1603–1611 (2011).
Bortesi, L. & Fischer, R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotech. Adv. 33, 41–52 (2015).
Sharwood, R. E., von Caemmerer, S., Maliga, P. & Whitney, S. M. The catalytic properties of hybrid Rubisco comprising tobacco small and sunflower large subunits mirror the kinetically equivalent source Rubiscos and can support tobacco growth. Plant Physiol. 146, 83–96 (2008).
Whitney, S. M. & Sharwood, R. E. Construction of a tobacco master line to improve Rubisco engineering in chloroplasts. J. Exp. Bot. 59, 1909–1921 (2008).
Blayney, M., Whitney, S. & Beck, J. NanoESI mass spectrometry of Rubisco and Rubisco activase structures and their interactions with nucleotides and sugar phosphates. J. Am. Soc. Mass. Spec. 22, 1588–1601 (2011).
Acknowledgements
We thank A. Cousins for supplying their S. viridis Rubisco kinetic data for analysis. This research was funded by the following grants from the Australian Research Council: DE130101760 (R.E.S.), DP120101603 (O.G., S.M.W.) and CE140100015 (O.G., S.M.W.).
Author information
Authors and Affiliations
Contributions
R.E.S., O.G. and S.M.W. designed the study and undertook the experimental work. M.V.K. undertook the phylogenetic analysis and L.H.G. the structural analysis. All authors contributed to drafting the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-7, Supplementary Tables 1-5, Supplementary References. (PDF 2566 kb)
Rights and permissions
About this article
Cite this article
Sharwood, R., Ghannoum, O., Kapralov, M. et al. Temperature responses of Rubisco from Paniceae grasses provide opportunities for improving C3 photosynthesis. Nature Plants 2, 16186 (2016). https://doi.org/10.1038/nplants.2016.186
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.186
This article is cited by
-
Understanding the biochemical, physiological, molecular, and synthetic biology approaches towards the development of C4 rice (Oryza sativa L.)
Cereal Research Communications (2024)
-
Grafting Rhodobacter sphaeroides with red algae Rubisco to accelerate catalysis and plant growth
Nature Plants (2023)
-
Correlative adaptation between Rubisco and CO2-concentrating mechanisms in seagrasses
Nature Plants (2022)
-
Targeted base editing in the plastid genome of Arabidopsis thaliana
Nature Plants (2021)
-
High photosynthetic capacity of Sahelian C3 and C4 plants
Photosynthesis Research (2021)