Abstract
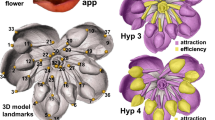
Polyploidy is an important driving force in angiosperm evolution, and much research has focused on genetic, epigenetic and transcriptomic responses to allopolyploidy. Nicotiana is an excellent system in which to study allopolyploidy because half of the species are allotetraploids of different ages, allowing us to examine the trajectory of floral evolution over time. Here, we study the effects of allopolyploidy on floral morphology in Nicotiana, using corolla tube measurements and geometric morphometrics to quantify petal shape. We show that polyploid morphological divergence from the intermediate phenotype expected (based on progenitor morphology) increases with time for floral limb shape and tube length, and that most polyploids are distinct or transgressive in at least one trait. In addition, we show that polyploids tend to evolve shorter and wider corolla tubes, suggesting that allopolyploidy could provide an escape from specialist pollination via reversion to more generalist pollination strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vanneste, K., Maere, S. & Van de Peer, Y. Tangled up in two: a burst of genome duplications at the end of the Cretaceous and the consequences for plant evolution. Phil. Trans. R. Soc. B 369, 20130353 (2014).
Soltis, D. E., Visger, C. J. & Soltis, P. S. The polyploidy revolution then…and now: Stebbins revisited. Am. J. Bot. 101, 1057–1078 (2014).
Jiao, Y. et al. A genome triplication associated with early diversification of the core eudicots. Genome Biol. 13, R3 (2012).
Jiao, Y. et al. Ancestral polyploidy in seed plants and angiosperms. Nature 473, 97–100 (2011).
Renny-Byfield, S. & Wendel, J. F. Doubling down on genomes: polyploidy and crop plants. Am. J. Bot. 101, 1711–1725 (2014).
Campbell, D. R., Waser, N. M. & Melendez-Ackerman, E. J. Analyzing pollinator-mediated selection in a plant hybrid zone: hummingbird visitation patterns on three spatial scales. Am. Nat. 149, 295–315 (1997).
Melendez-Ackerman, E. & Campbell, D. R. Adaptive significance of flower color and inter-trait correlations in an Ipomopsis hybrid zone. Evolution 52, 1293–1303 (1998).
Sletvold, N. & Agren, J. Pollinator-mediated selection on floral display and spur length in the orchid Gymnadenia conopsea. Int. J. Plant Sci. 9, 999–1009 (2010).
Fenster, C. B. Selection on floral morphology by hummingbirds. Biotropica 23, 98–101 (1991).
Castellanos, M. C., Wilson, P. & Thomson, J. D. ‘Anti-bee’ and ‘pro-bird’ changes during the evolution of hummingbird pollination in Penstemon flowers. J. Evol. Biol. 17, 876–885 (2004).
Gomez, J. M. et al. Spatial variation in selection on corolla shape in a generalist plant is promoted by the preference patterns of its local pollinators. Proc. R. Soc. B 275, 2241–2249 (2008).
Bissell, E. K. & Diggle, P. K. Modular genetic architecture of floral morphology in Nicotiana: quantitative genetic and comparative phenotypic approaches to floral integration. J. Evol. Biol. 23, 1744–1758 (2010).
Rieseberg, L. H. & Willis, J. H. Plant speciation. Science 317, 910–914 (2007).
Venail, J., Dell'Olivo, A. & Kuhlemeier, C. Speciation genes in the genus Petunia. Phil. Trans. R. Soc. B 365, 461–468 (2010).
Segraves, K. A. & Thompson, J. N. Plant polyploidy and pollination: floral traits and insect visits to diploid and tetraploid Heuchera grossulariifolia. Evolution 53, 1114–1127 (1999).
Clarkson, J. J. et al. Long-term genome diploidization in allopolyploid Nicotiana section Repandae (Solanaceae). New Phytol. 168, 241–252 (2005).
Leitch, I. J. et al. The ups and downs of genome size evolution in polyploid species of Nicotiana (Solanaceae). Ann. Bot. 101, 805–814 (2008).
Chase, M. W. et al. Molecular systematics, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae). Ann. Bot. 92, 107–127 (2003).
Clarkson, J. J., Kelly, L. J., Leitch, A. R., Knapp, S. & Chase, M. W. Nuclear glutamine synthetase evolution in Nicotiana: phylogenetics and the origins of allotetraploid and homoploid (diploid) hybrids. Mol. Phylogenet. Evol. 55, 99–112 (2010).
Clarkson, J. J. et al. Phylogenetic relationships in Nicotiana (Solanaceae) inferred from multiple plastid DNA regions. Mol. Phylogenet. Evol. 33, 75–90 (2004).
Kelly, L. J., Leitch, A. R., Clarkson, J. J., Knapp, S. & Chase, M. W. Reconstructing the complex origin of wild allotetraploid tobaccos (Nicotiana section Suaveolentes). Evolution 67, 80–94 (2013).
Bissell, E. K. & Diggle, P. K. Floral morphology in Nicotiana: architectural and temporal effects on phenotypic integration. Int. J. Plant Sci. 169, 225–240 (2008).
Kelly, L. J. et al. Intragenic recombination events and evidence for hybrid speciation in Nicotiana (Solanaceae). Mol. Biol. Evol. 27, 781–799 (2010).
Goodspeed, T. H. The Genus Nicotiana (Chronica Botanica, 1954).
Nilsson, L. A. The evolution of flowers with deep corolla tubes. Nature 334, 147–149 (1988).
Fulton, M. & Hodges, S. A. Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proc. R. Soc. Lond. B 266, 2247–2252 (1999).
Darwin, C. On the Various Contrivances by which British and Foreign Orchids are Fertilised by Insects (Murray, 1862).
Pauw, A., Stofberg, J. & Waterman, R. J. Flies and flowers and Darwin's race. Evolution 63, 268–279 (2009).
Whittall, J. B. & Hodges, S. A. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447, 706–709 (2007).
Cresswell, J. E. Stabilizing selection and the structural variability of flowers within species. Ann. Bot. 81, 463–473 (1998).
Nattero, J., Sérsic, A. N. & Cocucci, A. A. Patterns of contemporary phenotypic selection and flower integration in the hummingbird-pollinated Nicotiana glauca between populations with different flower–pollinator combinations. Oikos 119, 852–863 (2010).
Galen, C. & Cuba, J. Down the tube: pollinators, predators, and the evolution of flower shape in the alpine skypilot, Polemonium viscosum. Evolution 55, 1963–1971 (2001).
Temeles, E. J., Linhart, Y. B., Masonjones, M. & Masonjones, H. D. The role of flower width in hummingbird bill length-flower length relationships. Biotropica 34, 68–80 (2002).
Palacios-Vargas, J. G., Llampallas, J. & Hogue, C. L. Preliminary list of the insects and related terrestrial Athropoda of Socorro Island, Islas Revillagigedo, Mexico. Bull. South. Calif. Acad. Sci. 81, 138–147 (1982).
Hanna, G. D. Expedition to the Revillagigedo Islands, Mexico, in 1925. Proc. Calif. Acad. Sci. XV, 1–113 (1926).
Hahn, I. J., Hogeback, S., Romer, U. & Vergara, P. M. Biodiversity and biogeography of birds in Pacific Mexico along an isolation gradient from mainland Chamela via coastal Marias to oceanic Revillagigedo Islands. Vertebr. Zool. 62, 123–144 (2012).
Anthony, A. W. Avifauna of the Revillagigedo Islands. Auk 15, 311–318 (1898).
De Ibarra, N. H. & Giurfa, M. Discrimination of closed coloured shapes by honeybees requires only contrast to the long wavelength receptor type. Anim. Behav. 66, 903–910 (2003).
Giurfa, M., Eichmann, B. & Menzel, R. Symmetry perception in an insect. Nature 382, 458–461 (1996).
Herrera, C. M. Selection on complexity of corolla outline in a hawkmoth-pollinated violet. Evol. Trends Plants 7, 9–13 (1993).
Lehrer, M. & Campan, R. Generalization of convex shapes by bees: what are shapes made of? J. Exp. Biol. 208, 3233–3247 (2005).
Kelber, A. Innate preferences for flower features in the hawkmoth Macroglossum stellatarum. J. Exp. Biol. 200, 827–836 (1997).
Kaczorowski, R. L., Seliger, A. R., Gaskett, A. C., Wigsten, S. K. & Raguso, R. A. Corolla shape vs. size in flower choice by a nocturnal hawkmoth pollinator. Funct. Ecol. 26, 577–587 (2012).
Stang, M., Klinkhamer, P. G. L. & Van Der Meijden, E. Size constraints and flower abundance determine the number of interactions in a plant–flower visitor web. Oikos 112, 111–121 (2006).
Bond, W. J. Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Phil. Trans. R. Soc. Lond. B 344, 83–90 (1994).
Zayed, A. et al. Increased genetic differentiation in a specialist versus a generalist bee: implications for conservation. Conserv. Genet. 6, 1017–1026 (2005).
Johnson, S. D. & Steiner, K. E. Generalization versus specialization in plant pollination systems. Trends Ecol. Evol. 15, 140–143 (2000).
Wilson, P., Castellanos, M. C., Wolfe, A. D. & Thomson, J. D. in Plant–Pollinator Interactions: From Specialization to Generalization (eds Waser, N. M. & Ollerton, J. ) 47–68 (Univ. Chicago Press, 2006).
Tripp, E. A. & Manos, P. S. Is floral specialization an evolutionary dead-end? Pollination system transitions in Ruellia (Acanthaceae). Evolution 62, 1712–1736 (2008).
Day, E. H., Hua, X. & Bromham, L. Is specialization and evolutionary dead end? Testing for differences in speciation, extinction and trait transition rates across diverse phylogenies of specialists and generalists. J. Evol. Biol. 29, 1257–1267 (2016).
Vamosi, J. C., Armbruster, W. S. & Renner, S. S. Evolutionary ecology of specialization: insights from phylogenetic analysis. Proc. R. Soc. B 281, 20142004 (2014).
Rohlf, J. F. tps Utility Program (SUNY, 2006).
Rohlf, J. F. tpsDig Program (SUNY, 2006).
Rohlf, J. F. Relative Warps (SUNY, 2006).
ImageJ (US National Institutes of Health, 1997–2010).
Burk, L. G. Partial self-fertility in a theoretical amphiploid progenitor of N. tabacum. J Hered. 64, 348–350 (1973).
Moon, H. S., Nicholson, J. S. & Lewis, R. S. Use of transferable Nicotiana tabacum L. microsatellite markers for investigating genetic diversity in the genus Nicotiana. Genome 51, 547–559 (2008).
Clarkson, J. J. Nicotiana (Solanaceae): Insights from Molecular Phylogenetics and Cytogenetics PhD thesis, Queen Mary Univ. London (2006).
Anssour, S. et al. Phenotypic, genetic and genomic consequences of natural and synthetic polyploidization of Nicotiana attenuata and Nicotiana obtusifolia. Ann. Bot. 103, 1207–1217 (2009).
Acknowledgements
We thank K. Yoong Lim, A. Kovařík, C. Mhiri and I. T. Baldwin for providing us with synthetic polyploids and homoploids. This work was supported by the Natural Environment Research Council (NE/C511964/1 to A.R.L. and M.C.) and the Overseas Research Students Awards Scheme to E.W.M.
Author information
Authors and Affiliations
Contributions
E.M., M.C., S.K., A.R.L. and S.L. designed the research. E.M. collected the data and performed most of the analyses. S.L. performed analyses in Mathematica. E.M., A.R.L. and S.L. wrote the manuscript with help from M.C., S.K. and A.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-3 and Supplementary Table 1. (PDF 2193 kb)
Rights and permissions
About this article
Cite this article
McCarthy, E., Chase, M., Knapp, S. et al. Transgressive phenotypes and generalist pollination in the floral evolution of Nicotiana polyploids. Nature Plants 2, 16119 (2016). https://doi.org/10.1038/nplants.2016.119
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.119
This article is cited by
-
Frequent ploidy changes in Salicaceae indicates widespread sharing of the salicoid whole genome duplication by the relatives of Populus L. and Salix L.
BMC Plant Biology (2021)
-
Trait-dependent resemblance of the flowering phenology and floral morphology of the allopolyploid Cardamine flexuosa to those of the parental diploids in natural habitats
Journal of Plant Research (2020)
-
Early consequences of allopolyploidy alter floral evolution in Nicotiana (Solanaceae)
BMC Plant Biology (2019)
-
An extracted tetraploid wheat harbouring the BBAA component of common wheat shows anomalous shikimate and sucrose metabolism
BMC Plant Biology (2019)
-
The evolutionary significance of polyploidy
Nature Reviews Genetics (2017)