Abstract
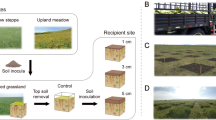
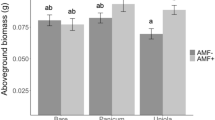
Many natural ecosystems have been degraded because of human activities1,2 and need to be restored so that biodiversity is protected. However, restoration can take decades and restoration activities are often unsuccessful3 because of abiotic constraints (for example, eutrophication, acidification) and unfavourable biotic conditions (for example, competition or adverse soil community composition). A key question is what manageable factors prevent transition from degraded to restored ecosystems and what interventions are required for successful restoration2,4. Experiments have shown that the soil community is an important driver of plant community development5–8, suggesting that manipulation of the soil community is key to successful restoration of terrestrial ecosystems3,9. Here we examine a large-scale, six-year-old field experiment on ex-arable land and show that application of soil inocula not only promotes ecosystem restoration, but that different origins of soil inocula can steer the plant community development towards different target communities, varying from grassland to heathland vegetation. The impact of soil inoculation on plant and soil community composition was most pronounced when the topsoil layer was removed, whereas effects were less strong, but still significant, when the soil inocula were introduced into intact topsoil. Therefore, soil inoculation is a powerful tool to both restore disturbed terrestrial ecosystems and steer plant community development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vitousek, P. M., Mooney, H. A., Lubchenco, J. & Melillo, J. M. Human domination of Earth's ecosystems. Science 277, 494–499 (1997).
Hobbs, R. J. & Harris, J. A. Restoration ecology: repairing the Earth's ecosystems in the new millennium. Restor. Ecol. 9, 239–246 (2001).
Eviner, V. T. & Hawkes, C. V. Embracing variability in the application of plant–soil interactions to the restoration of communities and ecosystems. Restor. Ecol. 16, 713–729 (2008).
Kulmatiski, A., Beard, K. H. & Stark, J. M. Soil history as a primary control on plant invasion in abandoned agricultural fields. J. Appl. Ecol. 43, 868–876 (2006).
Van der Putten, W. H. et al. Plant–soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265–276 (2013).
Kardol, P., Bezemer, T. M. & Van der Putten, W. H. Temporal variation in plant-soil feedback controls succession. Ecol. Lett. 9, 1080–1088 (2006).
Carbajo, V., den Braber, B., van der Putten, W. H. & De Deyn, G. B. Enhancement of late successional plants on ex-arable land by soil inoculations. PloS ONE 6, e21943 (2011).
De Deyn, G. B. et al. Soil invertebrate fauna enhances grassland succession and diversity. Nature 422, 711–713 (2003).
Harris, J. Soil microbial communities and restoration ecology: facilitators or followers? Science 325, 573–574 (2009).
Van der Wal, A. et al. Fungal biomass development in a chronosequence of land abandonment. Soil Biol. Biochem. 38, 51–60 (2006).
Bauer, J. T., Mack, K. M. L. & Bever, J. D. Plant-soil feedbacks as drivers of succession: evidence from remnant and restored tallgrass prairies. Ecosphere 6, art158–art158 (2015).
Tsiafouli, M. A. et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Change Biol. 21, 973–985 (2015).
Kulmatiski, A. & Beard, K. H. Long-term plant growth legacies overwhelm short-term plant growth effects on soil microbial community structure. Soil Biol. Biochem. 43, 823–830 (2011).
Jaunatre, R., Buisson, E. & Dutoit, T. Topsoil removal improves various restoration treatments of a Mediterranean steppe (La Crau, southeast France). Appl. Veg. Sci. 17, 236–245 (2014).
Marrs, R. H. in Advances in Ecological Research Vol. 24 (eds Fitter, A. H. & Begon, M. ) 241–300 (Academic Press, 1993).
Kardol, P., Bezemer, T. M. & van der Putten, W. H. Soil organism and plant introductions in restoration of species-rich grassland communities. Restor. Ecol. 17, 258–269 (2009).
Box, J. et al. Experimental wet heath translocation in Dorset, England. Ecol. Eng. 37, 158–171 (2011).
Bulot, A., Provost, E. & Dutoit, T. A comparison of different soil transfer strategies for restoring a Mediterranean steppe after a pipeline leak (La Crau plain, South-Eastern France). Ecol. Eng. 71, 690–702 (2014).
Vécrin, M. P. & Muller, S. Top-soil translocation as a technique in the re-creation of species-rich meadows. Appl. Veg. Sci. 6, 271–278 (2003).
Pywell, R. F., Meek, W. R., Webb, N. R., Putwain, P. D. & Bullock, J. M. Long-term heathland restoration on former grassland: The results of a 17-year experiment. Biol. Conserv. 144, 1602–1609 (2011).
Antonsen, H. & Olsson, P. A. Relative importance of burning, mowing and species translocation in the restoration of a former boreal hayfield: Responses of plant diversity and the microbial community. J. Appl. Ecol. 42, 337–347 (2005).
Hamman, S. T. & Hawkes, C. V. Biogeochemical and microbial legacies of non-native grasses can affect restoration success. Restor. Ecol. 21, 58–66 (2013).
Bakker, J. P. & Berendse, F. Constraints in the restoration of ecological diversity in grassland and heathland communities. Trends Ecol. Evol. 14, 63–68 (1999).
Bullock, J. M. Community translocation in Britain: Setting objectives and measuring consequences. Biol. Conserv. 84, 199–214 (1998).
Rowe, H. I., Brown, C. S. & Claassen, V. P. Comparisons of mycorrhizal responsiveness with field soil and commercial inoculum for six native montane species and Bromus tectorum. Restor. Ecol. 15, 44–52 (2007).
Emam, T. Local soil, but not commercial AMF inoculum, increases native and non-native grass growth at a mine restoration site. Restor. Ecol. 24, 35–44 (2016).
Paluch, E. C., Thomsen, M. A. & Volk, T. J. Effects of resident soil fungi and land use history outweigh those of commercial mycorrhizal inocula: Testing a restoration strategy in unsterilized soil. Restor. Ecol. 21, 380–389 (2013).
Hoeksema, J. D. et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 13, 394–407 (2010).
De Deyn, G. B., Raaijmakers, C. E. & Van der Putten, W. H. Plant community development is affected by nutrients and soil biota. J. Ecol. 92, 824–834 (2004).
van Elsas, J. D. et al. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl Acad. Sci. USA 109, 1159–1164 (2012).
Acknowledgements
We thank S. de Bruin, G. Heuvelink, and W. de Boer for their advice, and H. Hofman, H. Veerbeek, L. Veiken and A. Blankena for providing background information on the field site and the experiment. Thanks to R. Wagenaar, C. Raaijmakers, I. Chardon, H. Duyts, Q. Hakkaart, G. Bos, Y. Chau, M. Schrama, S. Schreven, J. Salamon, W. Dimmers, A. Moraru, H.-J. van der Kolk, L. Wit and I. Nugteren for technical assistance. This work was funded by the Netherlands Organization for Scientific Research (NWO ‘Biodiversiteit werkt’ project no. 841.11.008 to TMB). This is publication 6100 of the Netherlands Institute of Ecology (NIOO-KNAW).
Author information
Authors and Affiliations
Contributions
M.B., T.M.B. and W.H.v.d.P. initiated and designed the field experiment, M.B. implemented the field experiment, E.R.J.W. and T.M.B. designed the mesocosm experiment. E.R.J.W. collected and analysed the data and wrote the first draft. All authors contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Material
Supplementary Methods, Supplementary Figs 1–4, Supplementary Tables 1–9 and Supplementary References. (PDF 623 kb)
Rights and permissions
About this article
Cite this article
Wubs, E., van der Putten, W., Bosch, M. et al. Soil inoculation steers restoration of terrestrial ecosystems. Nature Plants 2, 16107 (2016). https://doi.org/10.1038/nplants.2016.107
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.107
This article is cited by
-
Soil microbial diversity plays an important role in resisting and restoring degraded ecosystems
Plant and Soil (2024)
-
Changes in composition and function of soil microbial communities during secondary succession in oldfields on the Tibetan Plateau
Plant and Soil (2024)
-
Experimental community coalescence sheds light on microbial interactions in soil and restores impaired functions
Microbiome (2023)
-
Leveraging microbiome rediversification for the ecological rescue of soil function
Environmental Microbiome (2023)
-
Above- and belowground fungal biodiversity of Populus trees on a continental scale
Nature Microbiology (2023)