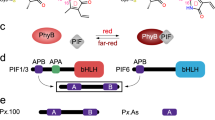
Abstract
Phytochromes are red/far-red-light detecting photoreceptors that regulate plant growth and development. They photo-interconvert between an inactive Pr (red-light absorbing) and a physiologically active Pfr (far-red-light absorbing) form, acting as light-controlled molecular switches. Although the two major plant phytochromes A (phyA) and B (phyB) share similar absorption properties, they exhibit dramatic differences in their action spectra. Since both phytochromes antagonistically regulate seedling development under vegetative shade, it is essential for plants to clearly distinguish between phyA and phyB action. This discrimination is not comprehensible solely by the molecular properties of the phytochromes, but is evidently due to the dynamics of the phytochrome system. Using an integrated experimental and mathematical modelling approach we show that phytochrome dimerization is an essential element for phyB function. Our findings reveal that light-independent Pfr to Pr relaxation (dark reversion) and association/dissociation to nuclear bodies (NBs) severely depend on the conformational state of the phyB dimer. We conclude that only Pfr–Pfr homodimers of phyB can be responsible for triggering physiological responses, leading to a suppression of phyB function in the far-red range of the light spectrum.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
17 October 2016
A Correction to this paper has been published: https://doi.org/10.1038/nplants.2016.47
References
Fankhauser, C. & Staiger, D. Photoreceptors in Arabidopsis thaliana: light perception, signal transduction and entrainment of the endogenous clock. Planta 216, 1–16 (2002).
Chen, M., Chory, J. & Fankhauser, C. Light signal transduction in higher plants. Annu. Rev. Genet. 38, 87–117 (2004).
Möglich, A., Yang, X., Ayers, R. A. & Moffat, K. Structure and function of plant photoreceptors. Annu Rev Plant Biol 61, 21–47 (2010).
Franklin, K. A. & Quail, P. H. Phytochrome functions in Arabidopsis development. J. Exp. Bot. 61, 11–24 (2010).
Casal, J. J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 64, 403–427 (2013).
Yanovsky, M. J., Casal, J. J. & Whitelam, G. C. Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ. 18, 788–794 (1995).
Rockwell, N. C., Su, Y.-S. & Lagarias, J. C. Phytochrome structure and signaling mechanisms. Annu. Rev. Plant Biol. 57, 837–858 (2006).
Chen, M., Schwab, R. & Chory, J. Characterization of the requirements for localization of phytochrome B to nuclear bodies. Proc. Natl Acad. Sci. USA 100, 14493–14498 (2003).
Fankhauser, C. & Chen, M. Transposing phytochrome into the nucleus. Trends Plant Sci. 13, 596–601 (2008).
Klose, C., Viczián, A., Kircher, S., Schäfer, E. & Nagy, F. Molecular mechanisms for mediating light-dependent nucleo/cytoplasmic partitioning of phytochrome photoreceptors. New Phytol. 206, 965–971 (2014).
Huq, E., Al-Sady, B. & Quail, P. H. Nuclear translocation of the photoreceptor phytochrome B is necessary for its biological function in seedling photomorphogenesis. Plant J. 35, 660–664 (2003).
Matsushita, T., Mochizuki, N. & Nagatani, A. Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature 424, 571–574 (2003).
Eichenberg, K. et al. Arabidopsis phytochromes C and E have different spectral characteristics from those of phytochromes A and B. FEBS Lett. 470, 107–112 (2000).
Smith, H., Xu, Y. & Quail, P. H. Antagonistic but complementary actions of phytochromes A and B allow seedling de-etiolation. Plant Physiol. 114, 637–641 (1997).
Rausenberger, J. et al. Photoconversion and nuclear trafficking cycles determine phytochrome A's response profile to far-red light. Cell 146, 813–825 (2011).
Rausenberger, J. et al. An integrative model for phytochrome B mediated photomorphogenesis from protein dynamics to physiology. PLoS ONE 5, e10721 (2010).
Yamaguchi, R., Nakamura, M., Mochizuki, N., Kay, S. A. & Nagatani, A. Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 145, 437–445 (1999).
Kircher, S. et al. Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 11, 1445–1456 (1999).
Kirchenbauer, D. Analysen zur intra-und interzellulären Phytochrom-Signalleitung. Dissertation (Albert-Ludwigs-Universität Freiburg, 2009).
Van Buskirk, E. K., Reddy, A. K., Nagatani, A. & Chen, M. Photobody localization of phytochrome B is tightly correlated with prolonged and light-dependent inhibition of hypocotyl elongation in the Dark. Plant Physiol. 165, 595–607 (2014).
Brockmann, J., Rieble, S., Kazarinova-Fukshansky, N., Seyfried, M. & Schäfer, E. Phytochrome behaves as a dimer in vivo. Plant Cell Environ. 10, 105–111 (1987).
Hennig, L. & Schäfer, E. Both subunits of the dimeric plant photoreceptor phytochrome require chromophore for stability of the far-red light-absorbing form. J. Biol. Chem. 276, 7913–7918 (2001).
Wagner, D., Tepperman, J. M. & Quail, P. H. Overexpression of phytochrome b induces a short hypocotyl phenotype in transgenic Arabidopsis. Plant Cell 3, 1275–1288 (1991).
Mira-Rodado, V. et al. Functional cross-talk between two-component and phytochrome B signal transduction in Arabidopsis. J. Exp. Bot. 58, 2595–2607 (2007).
Elich, T. D. & Chory, J. Biochemical characterization of Arabidopsis wild-type and mutant phytochrome B holoproteins. Plant Cell 9, 2271–2280 (1997).
Sweere, U. et al. Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 294, 1108–1111 (2001).
Jabben, M., Beggs, C. & Schäfer, E. Dependence of Pfr/Ptot-ratios on light quality and light quantity. Photochem. Photobiol. 35, 709–712 (1982).
Kretsch, T., Poppe, C. & Schäfer, E. A new type of mutation in the plant photoreceptor phytochrome B causes loss of photoreversibility and an extremely enhanced light sensitivity. Plant J. 22, 177–186 (2000).
Ádám, É. et al. Altered dark- and photoconversion of phytochrome B mediate extreme light sensitivity and loss of photoreversibility of the phyB-401 mutant. PLoS ONE 6, e27250 (2011).
Ni, W. et al. A mutually assured destruction mechanism attenuates light signaling in Arabidopsis. Science 344, 1160–1164 (2014).
Trupkin, S. A., Legris, M., Buchovsky, A. S., Tolava Rivero, M. B. & Casal, J. J. Phytochrome B nuclear bodies respond to the low red to far-red ratio and to the reduced irradiance of canopy shade in Arabidopsis. Plant Physiol. 165, 1698–1708 (2014).
Takala, H. et al. Signal amplification and transduction in phytochrome photosensors. Nature 509, 245–248 (2014).
Burgie, E. S., Bussell, A. N., Walker, J. M., Dubiel, K. & Vierstra, R. D. Crystal structure of the photosensing module from a red/far-red light-absorbing plant phytochrome. Proc. Natl Acad. Sci. USA 111, 10179–10184 (2014).
Medzihradszky, M. et al. Phosphorylation of phytochrome B inhibits light-induced signaling via accelerated dark reversion in Arabidopsis. Plant Cell 25, 535–544 (2013).
Botto, J. F. Sánchez, R. A. & Casal, J. J. Role of phytochrome B in the induction of seed germination by light in Arabidopsis thaliana. J. Plant Physiol. 146, 307–312 (1995).
Furuya, M. & Schäfer, E. Photoperception and signalling of induction reactions by different phytochromes. Trends Plant Sci. 1, 301–307 (1996).
VanDerWoude, W. J. A dimeric mechanism for the action of phytochrome: evidence from photothermal interactions in lettuce seed germination. Photochem. Photobiol. 42, 655–661 (1985).
Bauer, D. et al. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16, 1433–1445 (2004).
Hirschfeld, M., Tepperman, J. M., Clack, T., Quail, P. H. & Sharrock, R. A. Coordination of phytochrome levels in phyB mutants of Arabidopsis as revealed by apoprotein-specific monoclonal antibodies. Genetics 149, 523–535 (1998).
Dieterle, M., Zhou, Y. C., Schäfer, E., Funk, M. & Kretsch, T. EID1, an F-box protein involved in phytochrome A-specific light signaling. Genes Dev. 15, 939–944 (2001).
Kreutz, C., Raue, A., Kaschek, D. & Timmer, J. Profile likelihood in systems biology. FEBS J. 280, 2564–2571 (2013).
Acknowledgements
We thank Andreas Hiltbrunner, Jens Timmer and Robert Smith for helpful comments on the manuscript and Michael Volpers and Milad Adibi for helpful discussions and support. F.V. acknowledges funding from the LGFG/International Graduate Academy, University of Freiburg. C.K. was supported by DFG grant (SCHA 303/16-1) to E.S., S.K. acknowledges support by a DFG grant (KI 1077/2). C.F. was supported by the Human Frontier Science Program (HFSP Research Grant RGP0025/2013).
Author information
Authors and Affiliations
Contributions
C.F. and E.S. devised this project. C.K. designed and performed the experiments, except the nuclear body experiments and the hypocotyl measurements (Fig. 1). F.V. analysed the experimental data, performed all mathematical calculations, designed the nuclear body study and performed and analysed the nuclear body experiments. S.K. supervised the nuclear body study and performed some of these experiments (Supplementary Fig. 11). A.H. performed hypocotyl measurement (Fig. 1). E.S. supervised all other experiments; C.F. supervised the mathematical modelling. F.V., C.K., S.K., E.S. and C.F. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Klose, C., Venezia, F., Hussong, A. et al. Systematic analysis of how phytochrome B dimerization determines its specificity. Nature Plants 1, 15090 (2015). https://doi.org/10.1038/nplants.2015.90
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2015.90
This article is cited by
-
Reactive oxygen and nitrogen species (RONS) signalling in seed dormancy release, perception of environmental cues, and heat stress response
Plant Growth Regulation (2023)
-
Supplemental inter-lighting with additional far-red to red and blue light increases the growth and yield of greenhouse sweet peppers (Capsicum annuum L.) in winter
Horticulture, Environment, and Biotechnology (2023)
-
Plant phytochrome B is an asymmetric dimer with unique signalling potential
Nature (2022)
-
Plant multiscale networks: charting plant connectivity by multi-level analysis and imaging techniques
Science China Life Sciences (2021)
-
Increasing ambient temperature progressively disassembles Arabidopsis phytochrome B from individual photobodies with distinct thermostabilities
Nature Communications (2020)