Abstract
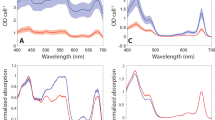
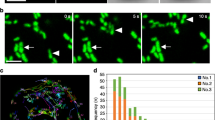
Life on earth relies upon photosynthesis, which consumes carbon dioxide and generates oxygen and carbohydrates. Photosynthesis is sustained by a dynamic environment within the plant cell involving numerous organelles with cytoplasmic streaming. Physiological studies of chloroplasts, mitochondria and peroxisomes show that these organelles actively communicate during photorespiration, a process by which by-products produced by photosynthesis are salvaged. Nevertheless, the mechanisms enabling efficient exchange of metabolites have not been clearly defined. We found that peroxisomes along chloroplasts changed shape from spherical to elliptical and their interaction area increased during photorespiration. We applied a recent femtosecond laser technology to analyse adhesion between the organelles inside palisade mesophyll cells of Arabidopsis leaves and succeeded in estimating their physical interactions under different environmental conditions. This is the first application of this estimation method within living cells. Our findings suggest that photosynthetic-dependent interactions play a critical role in ensuring efficient metabolite flow during photorespiration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hayashi, M. & Nishimura, M. Arabidopsis thaliana – a model organism to study plant peroxisomes. Biochim. Biophys. Acta 1763, 1382–1391 (2006).
Hu, J. et al. Plant peroxisomes: biogenesis and function. Plant Cell 24, 2279–2303 (2012).
Pracharoenwattana I. & Smith S. When is a peroxisome not a peroxisome? Trends Plant Sci. 13, 522–525 (2008).
Peterhansel, C. et al. Photorespiration. Arabidopsis Book 8, e0130 (2010).
Takahashi, S. & Badger, M. R. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci. 16, 53–60 (2011).
Kozaki, K. & Takeba, G. Photorespiration protects C3 plants from photooxidation. Nature 384, 557–560 (1996).
Hayashi, Y. et al. Direct interaction between glyoxysomes and lipid bodies in cotyledons of the Arabidopsis thaliana ped1 mutant. Protoplasma 218, 83–94 (2001).
Tolbert, N. E. & Essner, E. Microbodies: peroxisomes and glyoxysomes. J. Cell Biol. 91, 271s–283s (1981).
Beevers, H. Microbodies in higher plants. Annu. Rev. Plant Physiol. 30, 159–193 (1979).
Corpas, F. J. et al. Trends Plant Sci. 6, 145–150 (2001).
Spiess, G. M. & Zolma, B. K. Subcell. Biochem. 69, 257–281 (2013).
Wada, M., Kagawa, T. & Sato, Y. Chloroplast movement. Annu. Rev. Plant Biol. 54, 455–468 (2003).
Takagi, S., Islam, M. S. & Iwabuchi, K. Dynamic behavior of double-membrane-bounded organelles in plant cells. Int. Rev. Cell Mol. Biol. 286, 181–222 (2011).
Wada, M. & Suetsugu, N. Plant organelle positioning. Curr. Opin. Plant Biol. 7, 626–631 (2004).
Higaki, T., Sano, T. & Hasezawa, S. Actin microfilament dynamics and actin side-binding proteins in plants. Curr. Opin. Plant Biol. 10, 549–556 (2007).
Mathur, J., Mathur, N. & Hulskamp, M. Simultaneous visualization of peroxisomes and cytoskeletal elements reveals actin and not microtubule-based peroxisome motility in plants. Plant Physiol. 128, 1031–1045 (2002).
Mano, S. et al. Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: dynamic morphology and actin-dependent movement. Plant Cell Physiol. 43, 331–341 (2002).
Kaji, T. et al. Nondestructive micro-patterning of living animal cells using focused femtosecond laser-induced impulsive force. Appl. Phys. Lett. 91, 023904 (2007).
Hosokawa, Y. et al. Non-contact estimation of intercellular breaking force using a femtosecond laser impulse quantified by atomic force microscopy. Proc. Natl Acad. Sci. USA 108, 1777–1782 (2011).
Iino, T. & Hosokawa, Y. Direct measurement of femtosecond laser impulse in water by atomic force microscopy. Appl. Phys. Express 3, 107002 (2010).
Oikawa, K. et al. Chloroplast outer envelope protein CHUP1 is essential for chloroplast anchorage to the plasma membrane and chloroplast movement. Plant Physiol. 148, 829–842 (2008).
Vogel, A. & Venugopalan, V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 103, 577–644 (2003).
Vogel, A. et al. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 81, 1015–1047 (2005).
Usami, T. et al. Cryptochromes and phytochromes synergistically regulate Arabidopsis root greening under blue light. Plant Cell Physiol. 45, 1798–1808 (2004).
Ahmad, M. et al. Cryptochrome blue-light photoreceptors of Arabidopsis implicated in phototropism. Nature 392, 720–723 (1998).
Sakai, T. et al. Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl Acad. Sci. USA 98, 6969–6974 (2001).
Campbell, W. J. & Ogren, W. L. Electron transport through photosystem I stimulates light activation of ribulose bisphosphate carboxylase/oxygenase (Rubisco) by rubisco activase. Plant Physiol. 94, 479–484 (1990).
Murata, N. et al. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 1767, 414–421 (2007).
Braun, G. et al. Proton flow through the ATP synthase in chloroplasts regulates the distribution of light energy between PS I and PS II. FEBS Lett. 280, 57–60 (1991).
Kato, Y. et al. The variegated mutants lacking chloroplastic FtsHs are defective in D1 degradation and accumulate reactive oxygen species. Plant Physiol. 151, 1790–1801 (2009).
Hayashi, M. et al. AtPex14p maintains peroxisomal functions by determining protein targeting to three kinds of plant peroxisomes. EMBO J. 19, 5701–5710 (2000).
Takahashi, S., Bauwe, H. & Badger, M. Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiol. 144, 487–494 (2007).
Spector, I. et al. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science 219, 493–495 (1983).
Casella, J. F., Flanagan, M. D. & Lin, S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature 293, 302–305 (1981).
Oikawa, K. et al. Chloroplast unusual positioning is essential for proper chloroplast positioning. Plant Cell 15, 2805–2815 (2003).
Sheahan, M. B. et al. A green fluorescent protein fusion to actin-binding domain 2 of Arabidopsis fimbrin highlights new features of a dynamic actin cytoskeleton in live plant cells. Plant Physiol. 136, 3968–3978 (2004).
Ishijima, A. et al. Simultaneous observation of individual ATPase and mechanical events by a single myosin molecule during interaction with actin. Cell 92, 161–171 (1998).
Piramowicz, M. O. et al. Dynamic force measurements of avidin–biotin and streptavidin–biotin interactions using AFM. Acta Biochim. Polon. 53, 93–100 (2006).
Panorchan, P. et al. Single-molecule analysis of cadherin-mediated cell-cell adhesion. J. Cell Sci. 119, 66–74 (2006).
Sinclair, A. M., Trobacher, C. P., Mathur, N., Greenwood, J. S. & Mathur, J. Peroxule extension over ER-defined paths constitutes a rapid subcellular response to hydroxyl stress. Plant J. 59, 231–242 (2009).
Sunil, B. et al. Optimization of photosynthesis by multiple metabolic pathways involving interorganelle interactions: resource sharing and ROS maintenance as the bases. Photosynth. Res. 117, 61–71 (2013).
Hu, J. et al. A role for peroxisomes in photomorphogenesis and development of Arabidopsis. Science 297, 405–409 (2002).
Takemiya, A. et al. Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 17, 1120–1127 (2005).
Christie, J. M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 58, 21–45 (2007).
Peremyslov, V. V. et al. Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol. 146, 1109–1116 (2008).
Tominaga, M. et al. Higher plant myosin XI moves processively on actin with 35 nm steps at high velocity. EMBO J. 22, 1263–1272 (2003).
de Brito, O. M. & Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 (2008).
Binns, D. et al. An intimate collaboration between peroxisomes and lipid bodies. J. Cell Biol. 173, 719–731 (2006).
Kagawa, T. & Wada, M. Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol. 41, 84–93 (2000).
Arimura, S., Yamamoto, J., Aida, G. P., Nakazono, M. & Tsutsumi, N. Frequent fusion and fission of plant mitochondria with unequal nucleoid distribution. Proc. Natl Acad. Sci. USA 101, 7805–7808 (2004).
Acknowledgements
We thank Drs S. Arimura (The University of Tokyo) for providing the Mt-GFP plants, S. Takahashi (The Australian National University; ANU) for providing shmt1, and M.R. Badger (ANU) and S. Takahashi (ANU) for kindly discussing and suggesting experiments. This work was supported by MEXT KAKENHI (Grant-in-Aid for Scientific Research on Innovative Areas) to M.N. (no. 22120007) and Y.H. (no. 22120010).
Author information
Authors and Affiliations
Contributions
K.O., Y.H., and M.N. designed the entire study; K.O. performed most of the experiments; K.O., S. Matsunaga, S. Mano, K.Y., M.H., A.K., T.K., W.S., T.M., S.H., and M.W. performed the physiological and fluorescence microscopic experiments; Y.H., T.I., and A.S. performed the adhesion analysis using femtosecond laser and AFM; S. Matsunaga performed analyses of peroxisome movement; M.K. performed transmission electron microscopic analysis; K.O., Y.H., S. Mano, K.Y., and M.N. wrote the paper; Y.H. supervised femtosecond laser analysis; and M.N. supervised and supported the entire study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Oikawa, K., Matsunaga, S., Mano, S. et al. Physical interaction between peroxisomes and chloroplasts elucidated by in situ laser analysis. Nature Plants 1, 15035 (2015). https://doi.org/10.1038/nplants.2015.35
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2015.35
This article is cited by
-
Using the organelle glue technique to engineer the plant cell metabolome
Plant Cell Reports (2023)
-
Reactive oxygen and nitrogen species (RONS) signalling in seed dormancy release, perception of environmental cues, and heat stress response
Plant Growth Regulation (2023)
-
Pexophagy suppresses ROS-induced damage in leaf cells under high-intensity light
Nature Communications (2022)
-
Hydrodynamic particle focusing enhanced by femtosecond laser deep grooving at low Reynolds numbers
Scientific Reports (2021)
-
FMT, a protein that affects mitochondrial distribution, interacts with translation-related proteins in Arabidopsis thaliana
Plant Cell Reports (2021)