Abstract
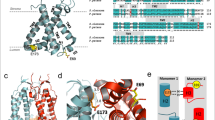
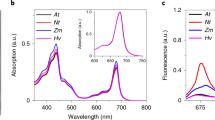
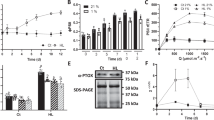
The non-photochemical quenching of light energy as heat (NPQ) is an important photoprotective mechanism that is activated in plants when light absorption exceeds the capacity of light utilization in photosynthesis. The PsbS protein plays a central role in this process and is supposed to activate NPQ through specific, light-regulated interactions with photosystem (PS) II antenna proteins. However, NPQ-specific interaction partners of PsbS in the thylakoid membrane are still unknown. Here, we have determined the localization and protein interactions of PsbS in thylakoid membranes in the NPQ-inactive (dark) and NPQ-active (light) states. Our results corroborate a localization of PsbS in PSII supercomplexes and support the model that the light activation of NPQ is based on the monomerization of dimeric PsbS and a light-induced enhanced interaction of PsbS with Lhcb1, the major component of trimeric light-harvesting complexes in PSII.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Møller, I. M., Jensen, P. E. & Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant. Biol. 58, 459–481 (2007).
Li, Z., Wakao, S., Fischer, B. B. & Niyogi, K. K. Sensing and responding to excess light. Annu. Rev. Plant. Biol. 60, 239–260 (2009).
Graßes, T. et al. The role of ΔpH-dependent dissipation of excitation energy in protecting photosystem II against light-induced damage in Arabidopsis thaliana. Plant Physiol. Biochem. 40, 41–49 (2002).
Roach, T. & Krieger-Liszkay, A. The role of the PsbS protein in the protection of photosystems I and II against high light in Arabidopsis thaliana. Biochim. Biophys. Acta 1817, 2158–2165 (2012).
Havaux, M. & Niyogi, K. K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl Acad. Sci. USA 96, 8762–8767 (1999).
Külheim, C., Agren, J. & Jansson, S. Rapid regulation of light harvesting and plant fitness in the field. Science 297, 91–93 (2002).
Külheim, C. & Jansson, S. What leads to reduced fitness in non-photochemical quenching mutants? Physiol. Plant. 125, 202–211 (2005).
Gollan, P. J., Tikkanen, M. & Aro, E. M. Photosynthetic light reactions: integral to chloroplast retrograde signalling. Curr. Opin. Plant Biol. 27, 180–191 (2015).
Tikkanen, M., Rantala, S. & Aro, E. M. Electron flow from PSII to PSI under high light is controlled by PGR5 but not by PSBS. Front. Plant Sci. 6, 521 (2015).
Nilkens, M. et al. Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim. Biophys. Acta 1797, 466–475 (2010).
Briantais, J. M., Vernotte, C., Picaud, M. & Krause, G. H. A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim. Biophys. Acta 548, 128–138 (1979).
Li, X. et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395 (2000).
Li, X. P. et al. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 279, 22866–22874 (2004).
Betterle, N. et al. Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J. Biol. Chem. 284, 15255–15266 (2009).
Holzwarth, A. R., Miloslavina, Y., Nilkens, M. & Jahns, P. Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem. Phys. Lett. 483, 262–267 (2009).
Horton, P., Wentworth, M. & Ruban, A. Control of the light harvesting function of chloroplast membranes: The LHCII-aggregation model for non-photochemical quenching. FEBS Lett. 579, 4201–4206 (2005).
Caffarri, S., Kouřil, R., Kereϊche, S., Boekema, E. J. & Croce, R. Functional architecture of higher plant photosystem II supercomplexes. EMBO J. 28, 3052–3063 (2009).
Nield, J., Funk, C. & Barber, J. Supermolecular structure of photosystem II and location of the PsbS protein. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1337–1344 (2000).
Fey, H. et al. Isolation of highly active photosystem II core complexes with a His-tagged Cyt b559 subunit from transplastomic tobacco plants. Biochim. Biophys. Acta 1777, 1501–1509 (2008).
Haniewicz, P. et al. Isolation of monomeric photosystem II that retains the subunit PsbS. Photosyn. Res. 118, 199–207 (2013).
Kiss, A. Z., Ruban, A. V. & Horton, P. The PsbS protein controls the organization of the photosystem II antenna in higher plant thylakoid membranes. J. Biol. Chem. 283, 3972–3978 (2008).
Kereϊche, S., Kiss, A. Z., Kouřil, R., Boekema, E. J. & Horton, P. The PsbS protein controls the macro-organisation of photosystem II complexes in the grana membranes of higher plant chloroplasts. FEBS Lett. 584, 759–764 (2010).
Bergantino, E. et al. Light- and pH-dependent structural changes in the PsbS subunit of photosystem II. Proc. Natl Acad. Sci. USA 100, 15265–15270 (2003).
Teardo, E. et al. Evidences for interaction of PsbS with photosynthetic complexes in maize thylakoids. Biochim. Biophys. Acta 1767, 703–711 (2007).
Aspinall-O'Dea, M. et al. In vitro reconstitution of the activated zeaxanthin state associated with energy dissipation in plants. Proc. Natl Acad. Sci. USA 99, 16331–16335 (2002).
Dominici, P. et al. Biochemical properties of the PsbS subunit of photosystem II either purified from chloroplast or recombinant. J. Biol. Chem. 277, 22750–22758 (2002).
Gerotto, C., Franchin, C., Arrigoni, G. & Morosinotto, T. In vivo identification of photosystem II light harvesting complexes interacting with photosystem II subunit S. Plant Physiol. 168, 1747–1761 (2015).
Wilka, L., Grunwald, M., Liaoc, P., Jomo Wallab, P. & Kühlbrandta, W. Direct interaction of the major light-harvesting complex II and PsbS in nonphotochemical quenching. Proc. Natl Acad. Sci. USA 110, 5452–5456 (2013).
Kouřil, R., Dekker, J. P. & Boekema, E. J. Supramolecular organization of photosystem II in green plants. Biochim. Biophys. Acta 1817, 2–12 (2012).
Johnson, M. P. et al. Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell 23, 1468–1479 (2011).
Horton, P. Optimization of light harvesting and photoprotection: molecular mechanisms and physiological consequences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 3455–3465 (2012).
Fan, M. et al. Crystal structures of the PsbS protein essential for photoprotection in plants. Nature Struct. Mol. Biol. 22, 729–735 (2015).
Pietrzykowska, M. et al. The light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis. Plant Cell 26, 3646–3660 (2014).
Li, X. P., Müller-Moulé, P., Gilmore, A. M. & Niyogi, K. K. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl Acad. Sci. USA 99, 15222–15227 (2002).
Schwarz, N. et al. Tissue-specific accumulation and regulation of zeaxanthin epoxidase in Arabidopsis reflect the multiple functions of the enzyme in plastids. Plant Cell Physiol. 56, 346–357 (2014).
Färber, A., Young, A. Y., Ruban, A., Horton, P. & Jahns, P. Dynamics of xanthophyll-cycle activity in different antenna subcomplexes in the photosynthetic membranes of higher plants (the relationship between zeaxanthin conversion and nonphotochemical fluorescence quenching). Plant Physiol. 115, 1609–1618 (1997).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Poschmann, G. et al. High-fat diet induced isoform changes of the Parkinson's disease protein DJ-1. J. Proteome Res. 13, 2339–2351 (2014).
Cox, J. et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MAXLFQ. Mol. Cell Proteomics 13, 2513–2526 (2014).
Pagliano, C. et al. Proteomic characterization and three-dimensional electron microscopy study of PSII-LHCII supercomplexes from higher plants. Biochim. Biophys. Acta 1837, 1454–1462 (2014).
Acknowledgements
This work was financially supported by the Deutsche Forschungsgemeinschaft (GRK 1525 to P.J.). We thank M. Benecke and K. Hoffie (IPK Gatersleben) for their highly skilled technical support.
Author information
Authors and Affiliations
Contributions
V.C.G., G.P. and P.J. designed the experiments; V.C.G. performed the experiments; G.P. and K.S. performed mass spectrometry and analysed data; M.M. performed the electron microscopy; V.C.G. and P.J. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Correa-Galvis, V., Poschmann, G., Melzer, M. et al. PsbS interactions involved in the activation of energy dissipation in Arabidopsis. Nature Plants 2, 15225 (2016). https://doi.org/10.1038/nplants.2015.225
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2015.225
This article is cited by
-
Recent progress in atomistic modeling of light-harvesting complexes: a mini review
Photosynthesis Research (2023)
-
A perspective on the major light-harvesting complex dynamics under the effect of pH, salts, and the photoprotective PsbS protein
Photosynthesis Research (2023)
-
Accessible versatility underpins the deep evolution of plant specialized metabolism
Phytochemistry Reviews (2023)
-
The PsbS protein and low pH are necessary and sufficient to induce quenching in the light-harvesting complex of plants LHCII
Scientific Reports (2021)
-
The molecular pH-response mechanism of the plant light-stress sensor PsbS
Nature Communications (2021)