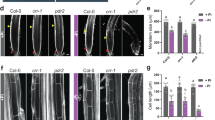
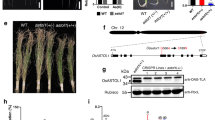
Abstract
Arsenic contamination of groundwater and soils threatens the health of tens of millions of people worldwide. Understanding the way in which arsenic is taken up by crops such as rice, which serve as a significant source of arsenic in the human diet, is therefore important. Membrane transport proteins that catalyse arsenic uptake by roots, and translocation through the xylem to shoots, have been characterized in a number of plants, including rice. The transporters responsible for loading arsenic from the xylem into the phloem and on into the seeds, however, are yet to be identified. Here, we show that transporters responsible for inositol uptake in the phloem in Arabidopsis also transport arsenic. Transformation of Saccharomyces cerevisiae with AtINT2 or AtINT4 led to increased arsenic accumulation and increased sensitivity to arsenite. Expression of AtINT2 in Xenopus laevis oocytes also induced arsenite import. Disruption of AtINT2 or AtINT4 in Arabidopsis thaliana led to a reduction in phloem, silique and seed arsenic concentrations in plants fed with arsenite through the roots, relative to wild-type plants. These plants also exhibited a large drop in silique and seed arsenic concentrations when fed with arsenite through the leaves. We conclude that in Arabidopsis, inositol transporters are responsible for arsenite loading into the phloem, the key source of arsenic in seeds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Smith, A., Lingas, E. & Rahman, M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull. World Health Organ. 78, 1093–1103 (2000).
Nordstrom, D. K. Public health-worldwide occurrences of arsenic in ground water. Science 296, 2143–2145 (2002).
Meharg, A. A. et al. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ. Sci. Technol. 43, 1612–1617 (2009).
Signes-Pastor, A. J. et al. Arsenic speciation in food and estimation of the dietary intake of inorganic arsenic in a rural village of West Bengal, India. J. Agric. Food Chem. 56, 9469–9474 (2008).
Li, G., Sun, G. X., Williams, P. N., Nunes, L. & Zhu, Y. G. Inorganic arsenic in Chinese food and its cancer risk. Environ. Int. 37, 1219–1225 (2011).
Meharg, A. A. & Zhao, F. J. Arsenic and Rice (Springer, 2012).
Shin, H., Shin, H. S., Dewbre, G. R. & Harrison, M. J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 39, 629–642 (2004).
Wu, Z. C., Ren, H. Y., McGrath, S. P., Wu, P. & Zhao, F. J. Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol. 157, 498–508 (2011).
Kamiya, T., Islam, M. R., Duan, G. L., Uraguchi, S. & Fujiwara, T. Phosphate deficiency signaling pathway is a target of arsenate and phosphate transporter OsPT1 is involved in As accumulation in shoots of rice. Soil Sci. Plant Nutr. 59, 580–590 (2013).
Sanders, O. I., Rensing, C., Kuroda, M., Mitra, B. & Rosen, B. P. Antimonite is accumulated by the glycerol facilitator GlpF in Escherichia coli. J. Bacteriol. 179, 3365–3367 (1997).
Liu, Z. et al. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl Acad. Sci. USA 99, 6053–6058 (2002).
Ma, J. F. et al. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl Acad. Sci. USA 105, 9931–9935 (2008).
Kamiya, T. et al. NIP1;1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. J. Biol. Chem. 284, 2114–2120 (2009).
Li, R. Y. et al. The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 150, 2071–2080 (2009).
Song, W. Y. et al. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl Acad. Sci. USA 111, 15699–15704 (2014).
Zhao, F. J., Ma, J. F., Meharg, A. A. & McGrath, S. P. Arsenic uptake and metabolism in plants. New Phytol. 181, 777–794 (2009).
Yamaji, N. & Ma, J. F. Further characterization of a rice silicon efflux transporter, Lsi2. Soil Sci. Plant Nutr. 57, 259–264 (2011).
Marschner, H. Mineral Nutrition of Higher Plants 2nd edn, 79–115 (Academic Press, 1995).
Carey, A. M. et al. Grain unloading of arsenic species in rice. Plant Physiol. 152, 309–319 (2010).
Carey, A. M. et al. Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytol. 192, 87–98 (2011).
Zheng, M. Z. et al. Spatial distribution of arsenic and temporal variation of its concentration in rice. New. Phytol. 189, 200–209 (2011).
Zhao, F. J., Stroud, J. L., Khan, M. A. & McGrath, S. P. Arsenic translocation in rice investigated using radioactive 73As tracer. Plant Soil. 350, 413–420 (2012).
Rothenberg, S. E., Mgutshini, N. L., Bizimis, M., Johnson-Beebout, S. E. & Ramanantsoanirina, A. Retrospective study of methylmercury and other metal(loid)s in Madagascar unpolished rice (Oryza sativa L.). Environ. Pollut. 196, 125–133 (2015).
Liu, Z., Boles, E. & Rosen, B. P. Arsenic trioxide uptake by hexose permeases in Saccharomyces cerevisiae. J. Biol. Chem. 279, 17312–17318 (2004).
Liu, Z. J., Styblo, M. & Rosen, B. P. Methylarsonous acid transport by aquaglyceroporins. Environ. Health Perspect. 114, 527–531 (2006).
Klepek, Y. S. et al. Arabidopsis POLYOL TRANSPORTER5, a new member of the monosaccharide transporter-like superfamily, mediates H+-symport of numerous substrates, including myo-inositol, glycerol, and ribose. Plant Cell 17, 204–218 (2005).
Joost, H. G. & Thorens, B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol. Membr. Biol. 18, 247–256 (2001).
Schneider, S. et al. Arabidopsis INOSITOL TRANSPORTER 4 mediates high-affinity H+ symport of myoinositol across the plasma membrane. Plant Physiol. 141, 565–577 (2006).
Schneider, S., Beyhl, D., Hedrich, R. & Sauer, N. Functional and physiological characterization of Arabidopsis INOSITOL TRANSPORTER1, a novel tonoplast-localized transporter for myo-inositol. Plant Cell 20, 1073–1087 (2008).
Schneider, S. et al. Arabidopsis INOSITOL TRANSPORTER2 mediates H+-symport of different inositol epimers and derivatives across the plasma membrane. Plant Physiol. 145, 1395–1407 (2007).
Nikawa, J., Tskugoshi, Y. & Yamashita, S. Isolation and characterization of two distinct myo-inositol transporter genes of Saccharomyces cerevisiae. J. Biol. Chem. 266, 11184–11191 (1991).
Ghosh, M., Shen, J. & Rosen, B. P. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl Acad. Sci USA 96, 5001–5006 (1999).
Luo, Y. et al. D-myo-inositol-3-phosphate affects phosphatidylinositol-mediated endomembrane function in Arabidopsis and is essential for auxin-regulated embryogenesis. Plant Cell 23, 1352–1372 (2011).
Fujiwara, T., Hirai, Y. M., Chino, M., Komeda, Y. & Naito, S. Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol. 99, 263–268 (1992).
Ishimaru, Y. et al. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 62, 379–390 (2010).
Zhu, Y. G. et al. High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environ. Sci. Technol. 42, 5008–5013 (2008).
Lalonde, S., Wipf, D. & Frommer, W. B. Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu. Rev. Plant Biol. 55, 341–372 (2004).
Schulz, A. in Plasmodesmata, Annual Plant Reviews Vol. 18 (ed. Oparka, K. J. ) 135–161 (Blackwell, 2005).
Turgeon, R. & Ayre, B. G. in Vascular Transport in Plants (eds Holbrook, N. M. & Zwieniecki, M. A. ) 45–67 (Elsevier/Academic Press, 2005).
Zhang, W. H. et al. Review: nutrient loading of developing seeds. Funct. Plant Biol. 34, 314–331 (2007).
Sauer, N. & Stolz, J. SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker′s yeast and identification of the histidine-tagged protein. Plant J. 6, 67–77 (1994).
Adams, A., Gottschling, D. E., Kaiser, C. & Stearns, T. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual (Cold Spring Harbor, 1998).
Hamdi, M. et al. Arsenic transport by zebrafish aquaglyceroporins. BMC Mol. Biol. 10, 104 (2009).
Tetyuk, O., Benning, U. F. & Hoffmann-Benning, S. Collection and analysis of Arabidopsis phloem exudates using the EDTA-facilitated method. J. Vis. Exp. 80, e51111 (2013).
Haslett, B. S., Reid, R. J. & Rengel, Z. Zinc mobility in wheat: uptake and distribution of zinc applied to leaves or roots. Ann. Bot. 87, 379–386 (2001).
Horie, T. et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 44, 928–938 (2005).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (41371458), the special fund for agro-scientific research in the public interest (201403015) to G.L.D. and Y.G.Z, and NIH grants R15 ES022800 to Z.L. and R37 GM55425 to B.P.R.
Author information
Authors and Affiliations
Contributions
Y.G.Z., Z.J.L., B.P.R. and N.S. designed the research. G.L.D., Y. H., S.S., J.M., J.C. and B.D. performed research and analysed data. All authors were involved in extensive discussions and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Duan, GL., Hu, Y., Schneider, S. et al. Inositol transporters AtINT2 and AtINT4 regulate arsenic accumulation in Arabidopsis seeds. Nature Plants 2, 15202 (2016). https://doi.org/10.1038/nplants.2015.202
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2015.202
This article is cited by
-
Current perspectives of ACR3 (arsenite efflux system) toward the reduction of arsenic accumulation in plants
Journal of Crop Science and Biotechnology (2024)
-
Exposure of Arsenic Associated with Cellular Turnover and Apoptosis Profile in the Bone Marrow of Mice Including Stem/Progenitor Population
Proceedings of the Zoological Society (2024)
-
Microbial biochemical pathways of arsenic biotransformation and their application for bioremediation
Folia Microbiologica (2023)
-
Arsenic in medicine: past, present and future
BioMetals (2023)
-
WRKY transcription factors: a promising way to deal with arsenic stress in rice
Molecular Biology Reports (2022)