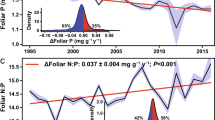
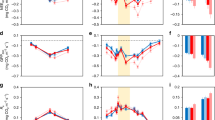
Abstract
Stimulation of forest productivity by elevated concentrations of CO2 is expected to partially offset continued increases in anthropogenic CO2 emissions. However, multiple factors can impair the capacity of forests to act as carbon sinks; prominent among these are tropospheric O3 and nutrient limitations1,2. Herbivorous insects also influence carbon and nutrient dynamics in forest ecosystems, yet are often ignored in ecosystem models of forest productivity. Here we assess the effects of elevated levels of CO2 and O3 on insect-mediated canopy damage and organic matter deposition in aspen and birch stands at the Aspen FACE facility in northern Wisconsin, United States. Canopy damage was markedly higher in the elevated CO2 stands, as was the deposition of organic substrates and nitrogen. The opposite trends were apparent in the elevated O3 stands. Using a light-use efficiency model, we show that the negative impacts of herbivorous insects on net primary production more than doubled under elevated concentrations of CO2, but decreased under elevated concentrations of O3. We conclude that herbivorous insects may limit the capacity of forests to function as sinks for anthropogenic carbon emissions in a high CO2 world.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Karnosky, D. F. et al. Free-Air exposure systems to scale up ozone research to mature trees. Plant Biol. 9, 181–190 (2007).
Reich, P. B. et al. Nitrogen limitation constrains sustainability of ecosystem response to CO2 . Nature 440, 922–925 (2010).
Bonan, G. B. Forests and climate change: forcings, feedbacks, and the climate benefit of forests. Science 320, 1444–1449 (2008).
Norby, R. J., Zak, D. R. Ecological lessons from Free-Air CO2 Enrichment (FACE) experiments. Annu. Rev. Ecol. Evol. Syst. 42, 181–203 (2011).
Karnosky, D. F. et al. Tropospheric ozone moderates responses of temperate hardwood forests to elevated CO2: a synthesis of molecular to ecosystem results from the Aspen FACE project. Funct. Ecol. 17, 289–304 (2003).
Ainsworth, E. A., Long, S. P. What have we learned from 15 years of free-air CO2 (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant productivity to rising CO2 . New Phytol. 165, 351–372 (2005).
Wittig, V. E., Ainsworth, E. A., Naidu, S. L., Karnosky, D. F., Long, S. P. Quantifying the impact of future tropospheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Global Change Biol. 15, 396–424 (2009).
Cole, C. T., Anderson, J. E., Lindroth, R. L., Waller, D. M. Rising concentrations of atmospheric CO2 have increased growth in natural stands of quaking aspen (Populus tremeuloides). Global Change Biol. 16, 2186–2197 (2010).
Fajvan, M. A., Wood, J. M. Stand structure and development after gypsy moth defoliation in the Appalachian Plateau. Forest Ecol. Manag. 89, 79–88 (1998).
Lovett, G. M. et al. Insect defoliation and nutrient cycling in forests. BioScience 52, 335–341 (2002).
Cook, B. D. et al. Using light-use and production efficiency models to predict photosynthesis and net carbon exchange during forest canopy disturbance. Ecosystems 11, 26–44 (2008).
Meehan, T. D., Couture, J. J., Bennett, A. B., Lindroth, R. L. Herbivore-mediated material fluxes in a northern deciduous forest under elevated carbon dioxide and ozone concentrations. New Phytol. 204, 397–407 (2014).
Couture, J. J., Meehan, T. D., Lindroth, R. L. Atmospheric change alters foliar quality of host trees and performance of two outbreak insect species. Oecologia 168, 863–876 (2012).
Couture, J. J., Lindroth, R. L. Atmospheric change alters performance of an invasive forest insect. Global Change Biol. 18, 3543–3557 (2012).
Hillstrom, M. L., Couture, J. J., Lindroth, R. L. Elevated carbon dioxide and ozone have weak, idiosyncratic effects on herbivorous forest insect abundance, species richness, and community composition. Insect Conserv. Diver. 7, 553–562 (2014).
Hillstrom, M. L., Lindroth, R. L. Elevated atmospheric carbon dioxide and ozone alter forest insect abundance and community composition. Insect Conserv. Diver. 1, 233–241 (2008).
Lindroth, R. L. Impacts of atmospheric CO2 and O3 on forests: phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 36, 2–21 (2010).
Hamilton, J. G. et al. Insect herbivory in an intact understory experimental CO2 enrichment. Oecologia 138, 566–573 (2004).
Dermondy, O. et al. Effects if elevated CO2 and O3 on leaf damage and insect abundance in a soybean agroecosystem. Arthropod Plant Interact. 2, 125–135.
Norby, R. J., Warren, J. M., Iversen, C. M., Medlyn, B. E., McMurtrie, R. E. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl Acad. Sci. USA 107, 19368–19373 (2010).
Lou, Y. et al. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. BioScience 54, 731–739 (2004).
Drake, J. E. et al. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol. Lett. 14, 349–357 (2011).
Zak, D. R., Pregitzer, K. S., Kubiske, M. E., Burton, A. J. Forest productivity under elevated CO2 and O3: positive feedbacks to soil N cycling sustain decade-long net primary productivity enhancement by CO2 . Ecol. Lett. 14, 1220–1226 (2011).
Phillips, R. P., Finzi, A. C., Bernhardt, E. S. Enhanced root exudation indices microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol. Lett. 14, 187–194 (2012).
Cramer, W. et al. Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Global Change Biol. 7, 357–373 (2001).
Stich, S. et al. Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon cycle feedbacks using five Dynamic Global Vegetation Models (DGVMs). Global Change Biol. 14, 2015–2039 (2008).
Zvereva, E. L., Zvereva, V., Kozlov, M. V. Little strokes fell great oaks: minor but chronic herbivory substantially reduces birch growth. Oikos 121, 2036–2043 (2012).
Talhelm, A. F. et al. Elevated carbon dioxide and ozone alter productivity and ecosystem carbon content in northern temperate forests. Global Change Biol. 20, 2492–2504 (2014).
Green, D. S., Erickson, J. E., Kruger, E. L. Foliar morphology and canopy nitrogen as predictors of light-use efficiency in terrestrial vegetation. Agr. Forest Meterol. 115, 163–171 (2003).
Gower, S. T., Kucharik, C. J., Norman, J. M. Direct and indirect estimation of leaf area index, fAPAR, and net primary production of terrestrial ecosystems. Remote Sens. Environ. 70, 29–51 (1999).
Acknowledgements
We are grateful to A. J. Burton for litter biomass data, K. S. Pregitzer, and A. F. Talhelm for LAI data, M. Kubiske for stand productivity data, P. A. Townsend for support to J.J.C. during the writing of this manuscript, and A. Gusse and K. F. Rubert-Nason for assistance with chemical analyses. Discussions with S. P. Serbin improved the content of the manuscript. Aspen FACE was principally supported by the Office of Science (BER), US Department of Energy Grant No. DE-FG02-95ER62125 to Michigan Technological University, Contract No. DE-AC02-98CH10886 to Brookhaven National Laboratory, the US Forest Service Northern Global Change Program and North Central Research Station, Michigan Technological University, and Natural Resources Canada – Canadian Forest Service. This research was supported by the Office of Science (BER), U.S. Department of Energy Grant No. DE-FG02-06ER64232 and University of Wisconsin Hatch grant WIS04898 to RLL and USDA NIFA AFRI Fellowship grant 2012-67012-19900 to J.J.C.
Author information
Authors and Affiliations
Contributions
R.L.L. designed the experiment and secured funding for the project; J.J.C. and T.D.M. collected field and laboratory data; J.J.C. and E.L.K. designed and performed the modelling exercise relating canopy damage to forest productivity; J.J.C. analysed the data and wrote the manuscript with the participation of R.L.L., T.D.M. and E.L.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Couture, J., Meehan, T., Kruger, E. et al. Insect herbivory alters impact of atmospheric change on northern temperate forests. Nature Plants 1, 15016 (2015). https://doi.org/10.1038/nplants.2015.16
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2015.16
This article is cited by
-
Host-specific growth responses of Larix kaempferi and Quercus acutissima to Asian gypsy moth defoliation in central Korea
Scientific Reports (2024)
-
Latitude, paleo-history and forest size matter for Afromontane canopy beetle diversity in a world context
Biodiversity and Conservation (2021)
-
Plant defences mediate interactions between herbivory and the direct foliar uptake of atmospheric reactive nitrogen
Nature Communications (2018)
-
Reflectance spectroscopy: a novel approach to better understand and monitor the impact of air pollution on Mediterranean plants
Environmental Science and Pollution Research (2018)
-
Effects of Elevated Atmospheric Carbon Dioxide and Tropospheric Ozone on Phytochemical Composition of Trembling Aspen ( Populus tremuloides ) and Paper Birch ( Betula papyrifera )
Journal of Chemical Ecology (2017)