Abstract
The childhood Asthma-Control Test (C-ACT) is validated for assessing asthma control in paediatric asthma. Among children aged 4–11 years, the C-ACT requires the simultaneous presence of both parent and child. There is an unmet need for a tool that can be used to assess asthma control in children when parents or caregivers are not present such as in the school setting. We assessed the psychometric properties and estimated the minimally important difference (MID) of the C-ACT and a modified version, comprising only the child responses (C-ACTc). Asthma patients aged 6–11 years (n=161) from a previously completed multicenter randomised trial were included. Demographic information, spirometry and questionnaire scores were obtained at baseline and during follow-up. Participants or their guardians kept a daily asthma diary. Internal consistency reliabilities of the C-ACT and C-ACTc were 0.76 and 0.67 (Cronbach’s α), respectively. Test–retest reliabilities of the C-ACT and C-ACTc were 0.72 and 0.66 (intra-class correlation), respectively. Significant correlations were noted between C-ACT scores and ACQ scores (Spearman’s correlation r=−0.56, 95% CI (−0.66, −0.44), P<0.001). The strength of the correlation between C-ACTc scores and ACQ scores was weaker (Spearman’s correlation r=−0.46, 95% CI (−0.58, −0.33), P<0.001). We estimated the MID for the C-ACT and C-ACTc to be 2 points and 1 point, respectively. Among asthma patients aged 6–11 years, the C-ACT had good psychometric properties. The psychometric properties of a shortened child-only version (C-ACTc), although acceptable, are not as strong.
Similar content being viewed by others
Introduction
One of the main goals of asthma therapy is to achieve and maintain good asthma control.1 Asthma control is best assessed using patient-reported outcomes.2 Unfortunately, there are a few validated instruments for use in paediatric populations. Among children of 5–11 years of age, the only measure of asthma control recommended by the 2010 National Institutes of Health (NIH) Asthma Outcomes Workshop is the childhood Asthma Control Test (C-ACT).3 The C-ACT is well validated for use among children aged 4–11 years.4 It is comprised of 3 parent-reported and 4 child-reported items and thus requires the simultaneous presence of both parent and child. There is need for an instrument that is suitable for use in settings where parents or caregivers are not readily accessible or may not be familiar with the child’s perception of disease burden.5 One important setting is in schools.6–10 Schools are often tasked with monitoring and assessing asthma in children, but there are no readily available tools for assessing asthma control.8–10 Such a tool may also be useful in a clinic setting when the guardian who accompanies the child to the visit might not have detailed knowledge about the child’s asthma. For example, many children spend time in several homes (mother, father, grandparents etc.) and may spend significant time away from home (school, daycare and after-school care). Therefore, the accompanying guardian may not have sufficient information to accurately complete asthma control questionnaires. At present, there are no validated alternatives to assess asthma control in children of 4–11 years of age in the absence of the parent or caregiver. Asthma diaries could serve as an alternative, especially if administered as a web-based diary.11,12 However, use of asthma diaries among younger patients in the absence of parents has not been demonstrated.
We examined the psychometric properties of the child responses (C-ACTc) and the full C-ACT, among paediatric patients enrolled in a multicenter clinical trial addressing the use of Lansoprazole in children with poor asthma control and without symptomatic gastroesophageal reflux (GER; NCT00442013).13 We also obtained a preliminary estimate of the minimally important difference (MID) for the C-ACT and C-ACTc.
Results
Study population
Data from 161 study participants aged 6–11 years from the SARCA trial were included (Table 1). The mean age of these participants was 9 years (s.d., 1.6). A majority were male (63%) and black (50%). Twenty-eight per cent and 18% of the participants were white and Hispanic, respectively. At baseline, 51% of study participants reported using combination inhaled corticosteroid/long-acting β2 treatment within the past 6 months and 74% reported use of systemic corticosteroids for asthma within the past year.
Reliability
At baseline, the internal consistency reliability (Cronbach’s α) was 0.76 for the C-ACT and 0.67 for the C-ACTc. The test–retest reliability (ICC) for C-ACT scores between two consecutive visits among subjects with stable asthma ranged from 0.44 to 0.94, with an average of 0.72 (Table 2). For the C-ACTc, the range was 0.37 to 0.88, with an average of 0.66. ICCs for both C-ACT and C-ACTc improved over time.
Construct validity
For the C-ACT, statistically significant Spearman’s correlations were observed between baseline C-ACT scores and baseline ACQ, baseline ASUI scores and pAQLQ. For the C-ACTc, statistically significant Spearman’s correlations were also observed between baseline C-ACTc scores and other asthma questionnaires: ACQ, ASUI and pAQLQ (Table 3).
Responsiveness
Mean C-ACT scores were significantly lower, indicating worse asthma control, among participants who experienced an episodes of poor asthma control (EPAC) when compared with those who did not (Table 4). The mean difference in C-ACT score between participants with an EPAC versus those without an EPAC was 1.6 points (95% CI (1.0, 2.0), P<0.001). Similarly, the mean C-ACTc scores were significantly lower, among patients who had experienced an EPAC when compared with those who did not (Table 5). The mean difference in C-ACTc score between participants with an EPAC versus those without an EPAC was 0.38 points (95% CI (0.14, 0.63), P<0.01).
The C-ACT and C-ACTC scores distinguished between groups with good control versus those with poor control. As hypothesised, C-ACT and C-ACTc scores improved among participants who experienced an improvement in asthma control. For those who experienced worsening control, the C-ACT and C-ACTc scores decreased. However, these changes were not statistically significant for the C-ACTc. (Table 6 and Supplementary Table 1).
MID
We used two distribution-based approaches to estimate the MID for the C-ACT and C-ACTc.14,15 Therefore, based on the s.d. of 4.1, and the s.e.m. of 2 for the C-ACT in our study population, we estimated the MID for the C-ACT to be 2 points. Using the anchor-based approach, we determined a mean difference in C-ACT scores between visits with an EPAC in the prior period and visits without an EPAC of 1.56 points. By rounding up to the nearest digit, the MID for the C-ACT was estimated to be 2 points. In adults, the MID for the ACT questionnaire has been estimated to be 3 points.16
The s.d. and s.e.m. of the C-ACTc were 2.2 and 1.26, respectively. By using the distribution-based approach, we estimated the MID for the C-ACTc in our study population to be 1 point. With the anchor-based approach, we determined the mean difference in C-ACTc scores between visits with an EPAC in the prior period and visits without an EPAC to equal 0.56 points. By rounding up to the nearest digit, we estimated the MID for the C-ACTc at 1 point.
Discussion
Main findings
The main findings of this study are that the C-ACT questionnaire has good psychometric properties in a population of paediatric patients with poorly controlled asthma. The psychometric properties of a shortened version with only the responses of the child, the C-ACTc, although acceptable, are not as good. We estimated the MID for the C-ACT and the C-ACTc to be 2 points and 1 point, respectively. The estimate of MID for the C-ACTc is a relatively larger change and needs further validation. The reliability coefficient for the C-ACTc was 0.67 compared with 0.76 for the C-ACT. Generally, values of 0.70–0.95 are considered acceptable; however, the observed lower α could be because of the shorter number of questions in the C-ACTc. At a group level, both questionnaires distinguished between patients with poor control versus those with good asthma control. We also found that for both the C-ACT and C-ACTc, ICCs improve over time which suggests that there may be a learning curve for the children. However, this does not affect the interpretation of the psychometric properties as both questionnaires showed responsiveness to changes in asthma control.
Strengths and limitations of this study
The strengths of this analysis include the following: the use of robust statistical methods to assess the psychometric properties and to estimate the minimal important difference of the C-ACT and C-ACTc; and the inclusion of a group of well-characterised patients who were prospectively recruited for an asthma intervention study. However, there are several notable limitations. First, in assessing the shortened version (C-ACTc), we recognise that even though the C-ACT instructions indicate that the parents should not help the child complete their section of the questionnaire, it is possible that some of the child responses might be influenced by the parent or caregiver present. Second, we demonstrated an ability to differentiate people with good and bad control based on EPAC analysis but did not demonstrate responsiveness. The C-ACT looked like it could capture improvement but not decline within the range of change that occurred in this cohort. Responsiveness to change at the individual level is best assessed with a prospective design using provider assessment of change in asthma control as an anchor. Therefore, in this post hoc analysis of the data collected as part of a randomised control trial, we were unable to assess responsiveness to change in C-ACT and C-ACTc scores at the individual level. In addition, our study population was restricted to patients with poorly controlled asthma. Without a full spectrum of disease severity, it is difficult to accurately assess responsiveness at the individual level. Another limitation of this study is the use of EPACs as anchors to determine the MID of the C-ACT. We did not have data on other anchors such as physician’s global rating of change in asthma control or the patient’s subjective assessment of change in asthma control over time. However, information about EPACs was prospectively collected with asthma diaries. The external validity of the study to children with mild or controlled asthma is limited because only patients with poorly controlled asthma were included and also because our study population had a relatively high representation of African–American children (50%).
Interpretation of findings in relation to previously published work
Liu et al.4 have previously shown that C-ACT scores have good internal consistency reliability, test–retest reliability and construct validity in paediatric asthma. Asthma is characterised by significant variations in daily symptoms, and the data on asthma morbidity can be captured using daily asthma diaries. However, asthma diaries are burdensome, especially for children. The ability to recall and discriminate differences in asthma symptoms is also likely to be highly variable among children. One study compared daily diary report of symptoms with the Pediatric Asthma Health Outcome Measure (PAHOM)17 questionnaire recall among elementary school children and found poor agreement after two days.18 Children underreported asthma symptoms on the PAHOM questionnaire when compared with those in diary entries.18 Other studies have confirmed the weak agreement between parent and child responses to asthma questionnaires.5,19–23 Inclusion of only the child-reported items in the C-ACT questionnaire is meant to measure the disease burden from the child’s point of view.
Implications for future research, policy and practice
There is an unmet need for a tool that can be used to assess asthma control in children when parents or caregivers are not present, such as in the school setting or in a clinical setting, as there are times when the guardian who accompanies the child to the visit might not have much detailed knowledge about the child’s asthma. The C-ACTc is therefore a promising instrument for assessing asthma control in these settings. We recognise that the child-reported items were originally designed as part of the C-ACT questionnaire and not intended to be scored separately. However, possible improvements to validating the C-ACTc would include: review of the face validity, a prospective study design with provider and parent rating of change in asthma control as an anchor, including asthma patients with a broad spectrum of asthma severity and control, and making sure that the questionnaire is administered to the child in the absence of the parent or caregiver as would be the case in a school setting. Alternatively, an instrument to meet this need could be developed from the start with item generation, selection, reduction and validation. Although this might delay the availability of an acceptable child-only instrument, it may be possible to improve upon general test characteristic of the instrument.
Conclusions
In summary, we showed that among asthma patients aged 6–11 years, the C-ACT has good psychometric properties and a shortened version with only the responses of the child, the C-ACTc is promising but needs additional study before it can be used in scientific research and daily practice. We also estimated the MID for the C-ACT and C-ACTc in our study population to be 2 points and 1 point, respectively.
Materials and Methods
Data collection
Patients
Data from 161 asthma patients 6–11 years of age, enrolled in the Study of Acid Reflux in Children with Asthma (SARCA) clinical trial (NCT00442013) were included in this analysis.13 The SARCA clinical trial was conducted by the American Lung Association Asthma Clinical Research Centers (ALA-ACRC) network from April 2007 to September 2011. Among other exclusion criteria, patients were also excluded if they had a history of neonatal respiratory distress or premature birth at less than 33 weeks' gestational age, had a forced expiratory volume in the first second (FEV1) of less than 60% predicted, or were non-adherent (<80% completion of daily diaries during run-in).13 Prior to enrolment, all study participants were treated with inhaled corticosteroids (⩾176 μg d−1 of fluticasone equivalents) and had no change in controller therapies for at least 8 weeks.13
Procedures
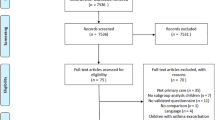
In the SARCA trial (N=306), baseline demographic data, spirometry, Asthma Control Questionnaire (ACQ)24 score, C-ACT score (ages 6–11 years), ACT score (ages 12–17 years), Asthma Symptom Utility Index (ASUI)25 score and the Pediatric Asthma Quality of Life Questionnaire (pAQLQ)26 score were obtained. It should be noted that participants were enrolled for the study only if they had an ACQ score of 1.25 or higher. They were then randomised to either lansoprazole (15 mg per day for children weighing <30 kg; 30 mg per day for children weighing ⩾30 kg) or matching placebo in addition to their inhaled corticosteroids. Participants returned to clinical centres every 4 weeks for evaluation that included spirometry and questionnaire scores. Participants also completed a daily dairy documenting daily morning peak expiratory flow, daily asthma symptoms scores, β2 use, nocturnal asthma awakenings, asthma treatments and healthcare use. Asthma diaries were collected at subsequent visits that occurred every 4 weeks. There were a total of six monthly follow-up visits. Our analysis focused on 161 children aged 6–11 years who met eligibility criteria. We excluded 102 participants aged 12 years or older. Figure 1 shows the details of participant flow during the study.
Measures
C-ACT
The C-ACT is a seven-item questionnaire that was developed and validated to assess asthma control among children 4–11 years old.4 Four questions are answered by the child and three by the parent or caregiver. The four child-reported items are scored on a 4-point numeric scale from 0 to 3 with higher numbers indicating better asthma control. One question asks, ‘How is your asthma today.’ The remaining 3 ask about activity limitation, cough and nighttime awakenings without a clear recall period. The 3 parent-reported items are scored on a 6-point numeric scale from 0 to 5, with higher numbers indicating better asthma control. Parents are asked about daytime symptoms, wheeze, and nighttime awakenings. The adult items are based on a recall period of 4 weeks. The final score of the C-ACT is the simple sum of each item and ranges from 0 to 27. Higher scores indicate better asthma control.
C-ACTc
The C-ACTc is comprised of only the original 4 child-reported items. The score ranges from 0 to 12 with higher scores indicating better asthma control.
ACQ-6
The ACQ-6 is well validated for assessing asthma control among patients older than 6 years.24 Items ask about nighttime awakenings, morning symptoms, activity limitations, breathlessness, wheezing and short-acting bronchodilator use. The ACQ-6 has a recall period of one week. The scores range from 0 to 6, and higher scores indicate worse control.
ASUI
The ASUI is a 10-item questionnaire, with a 2-week recall period, designed to assess the frequency and severity of four asthma symptoms (cough, wheeze, dyspnoea and awakening at night) and side effects of asthma medications.25 The scores range from 0 to 1 with higher scores indicating fewer asthma symptoms.
pAQLQ
The pAQLQ is a 23-item questionnaire that measures asthma-related functional impairments in patients 7–17 years old and comprises three domains: symptoms; activity limitation and emotional function.26 The pAQLQ has a recall period of one week. The scores range from 1 to 7, and higher scores indicate better asthma control.
Episodes of poor asthma control
Participants or their parents (for those ages 6–10 years) kept daily asthma diaries documenting asthma symptom scores, nocturnal asthma awakenings, frequency of β-agonist use, morning peak expiratory flow rate (PEFR), asthma treatments and asthma-related healthcare use. Using information obtained from asthma diaries, patients were monitored for the occurrence of EPACs. An EPAC was defined as the occurrence of at least one of the following events: an increase in rescue medication use over baseline (either ⩾4 additional puffs of bronchodilator for asthma symptoms or ⩾2 additional nebuliser treatments in 1 day), the occurrence of an unscheduled contact with a healthcare provider for asthma, use of systemic corticosteroids for asthma, or a decrease of 30% or more in morning PEFR on two consecutive days, as compared with the patient’s best PEFR during the run-in period.13
Spirometry
Pre- and post-bronchodilator spirometry was obtained according to American Thoracic Society (ATS) standards using the KoKo spirometer.27
Statistical analysis
All statistical analyses were conducted for the C-ACT and the C-ACTc separately. Asthma diary card data were used to evaluate the patient’s asthma control for the period between visits. The C-ACT and C-ACTc scores at the end of the period or the change in scores between visits were used as dependent variables in each case. For analyses that included multiple measurements from patients, generalised estimating equations (GEE) were used to account for the correlation between repeated measures from the same patient.28 To evaluate the internal consistency reliability, the Cronbach α coefficients were calculated using baseline C-ACT and C-ACTc scores. The Cronbach’s α coefficient measures the degree to which items on a questionnaire measure the same unidirectional construct. To assess the test–retest reliability, we calculated the intra-class correlation coefficient (ICC) of questionnaire scores between consecutive visits, four weeks apart among patients with stable asthma. Stable asthma was defined by the absence of an EPAC between 2 consecutive visits. Construct validity was assessed by computing Spearman’s rank correlations between C-ACT and C-ACTc scores at the baseline visit with ACQ, ASUI and pAQLQ scores. The ability of the C-ACT and C-ACTc to discriminate between patient groups with different levels of asthma control was assessed by comparing mean C-ACT and C-ACTc scores across groups with or without an EPAC or an EPAC component in the prior period. Analysis of variance with adjustment for repeated measures was used to compare mean C-ACT and C-ACTc scores between the patient groups. On the basis of the occurrence of EPACS during follow-up, we defined four levels of asthma control: good control, no events between visits; worsening control, good control in the previous 4-week period followed by an event during the 4-week period before the next visit; improved control, an event in the previous 4-week period (prior period) but no event in the subsequent 4-week period; and continuing poor control, an event in the prior period and a subsequent 4-week period.13 Responsiveness of the C-ACT and C-ACTc was assessed by comparing the mean changes in scores from beginning to the end of each period, across the 4 levels of control using analysis of variance with adjustment for repeated measures. Mean C-ACT and C-ACTc scores were compared by means of analysis of variance between patient groups who did or did not have an EPAC.
We used both the distribution- and anchor-based methods to determine the MID for the C-ACT and the C-ACTc.29–33 For the distribution-based approach, baseline data were used to determine the s.d. and the standard error of the mean (s.e.m.) of the C-ACT and the C-ACTc. The s.e.m. was computed using the formula below where reliability corresponded to the Cronbach α coefficient for the C-ACT and C-ACTc.
, .
By convention, the MID is calculated as 0.5×s.d.14 or 1×s.e.m.15 For the anchor-based approach, the 4 components that constituted an EPAC were used as anchors for determining change in asthma control. The mean C-ACT and C-ACTc scores between visits with an EPAC in the prior period and those without an EPAC in the prior period were calculated using GEE methods. The difference in mean scores was computed; the arithmetic average of these differences corresponded to the MID. Data were analysed using SAS version 9.0 (SAS Institute Inc.).
References
Expert Panel Report 3 (EPR-3). Guidelines for the Diagnosis and Management of Asthma, Summary Report 2007. National Asthma Education and Prevention Program Expert Panel Report 3. 120(Supplement 1): S94-S138 (2007).
Busse W. W., Morgan W. J., Taggart V. & Togias A . Asthma outcomes workshop: overview. Standardizing Asthma Outcomes in Clinical Research: Report of the Asthma Outcomes Workshop 129(3, Supplement): S1–S8 (2012).
Cloutier M. M. et al. Asthma outcomes: Composite scores of asthma control. Standardizing Asthma Outcomes in Clinical Research: Report of the Asthma Outcomes Workshop 129(3, Supplement): S24–S33 (2012).
Liu A. H. et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J. Allerg. Clin. Immunol. 119, 817–825 (2007).
Gerald, J. K. et al. Measurement characteristics of the pediatric asthma health outcome measure. J. Asthma 49, 260–266 (2012).
Mangan, J. M. & Gerald, L. B. Asthma agents: monitoring asthma in school. J. School Health 76, 300–302 (2006).
Wittich, A. R., Li, Y. & Gerald, L. B. Comparison of parent and student responses to asthma surveys: students grades 1-4 and their parents from an urban public school setting. J. School Health 76, 236–240 (2006).
Gerald L. B. et al. Increasing adherence to inhaled steroid therapy among schoolchildren: randomized, controlled trial of school-based supervised asthma therapy. Pediatrics 123, 466–474 (2009).
Gerald, L. B. et al. Can a school-based hand hygiene program reduce asthma exacerbations among elementary school children? J. Allergy Clin. Immunol. 130, 1317–1324 (2012).
Grad, R. et al. Peak flow measurements in children with asthma: what happens at school? J. Asthma 46, 535–540 (2009).
Voorend-van Bergen S. et al. Monitoring childhood asthma: web-based diaries and the asthma control test. The Journal of allergy and clinical immunology 133, 1599–1605.e1592 (2014).
Bime, C., Nguyen, J. & Wise, R. A. Measures of asthma control. Current opinion in pulmonary medicine 18, 48–56 (2012).
American Lung Association Asthma Clinical Research Centers W. Lansoprazole for children with poorly controlled asthma: A randomized controlled trial. JAMA 307, 373–380 (2012).
Norman, G. R., Sloan, J. A. & Wyrwich, K. W. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med. Care 41, 582–592 (2003).
Wyrwich, K. W., Tierney, W. M. & Wolinsky, F. D. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J. Clin. Epidemiol. 52, 861–873 (1999).
Schatz, M. et al. The minimally important difference of the Asthma Control Test. J. Allergy Clin. Immunol. 124, 719–723.e711 (2009).
Chiou, C. F., Weaver, M. R., Bell, M. A., Lee, T. A. & Krieger, J. W. Development of the multi-attribute Pediatric Asthma Health Outcome Measure (PAHOM). Int. J. Qual. Health Care 17, 23–30 (2005).
Joe K. G., Kathleen F. H., Leslie M., William C. B. & Lynn B. G . Innovative Approaches And New Developments In Lung Health Research A5805-A5805 (American Thoracic Society, 2010).
Theunissen, N. C. et al. The proxy problem: child report versus parent report in health-related quality of life research. Qual. Life Res. 7, 387–397 (1998).
Lara, M. et al. Differences between child and parent reports of symptoms among Latino children with asthma. Pediatrics 102, E68 (1998).
le Coq, E. M., Boeke, A. J., Bezemer, P. D., Colland, V. T. & van Eijk, J. T. Which source should we use to measure quality of life in children with asthma: the children themselves or their parents? Qual. Life Res. 9, 625–636 (2000).
Hedman, L., Lindgren, B., Perzanowski, M. & Ronmark, E. Agreement between parental and self-completed questionnaires about asthma in teenagers. Pediatr. Allergy Immunol. 16, 176–181 (2005).
Magzamen, S., Mortimer, K. M., Davis, A. & Tager, I. B. School-based asthma surveillance: a comparison of student and parental report. Pediatr. Allergy Immunol. 16, 669–678 (2005).
Juniper, E. F., O'Byrne, P. M., Guyatt, G. H., Ferrie, P. J. & King, D. R. Development and validation of a questionnaire to measure asthma control. Eur. Respir. J. 14, 902–907 (1999).
Revicki, D. A., Leidy, N. K., Brennan-Diemer, F., Sorensen, S. & Togias, A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest 114, 998–1007 (1998).
Juniper, E. F. et al. Measuring quality of life in the parents of children with asthma. Qual. Life Res. 5, 27–34 (1996).
Miller, M. R. et al. Standardisation of spirometry. Eur. Respir. J. 26, 319–338 (2005).
Hardin, J. & Hilbe, J. Generalized Estimating Equations (Chapman and Hall/CRC, 2003).
Guyatt, G. H., Osoba, D., Wu, A. W., Wyrwich, K. W. & Norman, G. R. Clinical Significance Consensus Meeting G. Methods to Explain the Clinical Significance of Health Status Measures. Mayo Clin. Proc. 77, 371–383 (2002).
Jaeschke, R., Singer, J. & Guyatt, G. H. Measurement of health status: Ascertaining the minimal clinically important difference. Control. Clin. Trials 10, 407–415 (1989).
Hays, R. D., Farivar, S. S. & Liu, H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD: J. Chron. Obstruct. Pulm. Dis. 2, 63–67 (2005).
Revicki, D., Hays, R. D., Cella, D. & Sloan, J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J. Clin. Epidemiol. 61, 102–109 (2008).
Yost, K. J. & Eton, D. T. Combining distribution- and anchor-based approaches to determine minimally important differences. Eval. Health Prof. 28, 172–191 (2005).
Acknowledgements
Investigators and Staff of the American Lung Association Asthma Clinical Research Centers: Baylor College of Medicine: Nicola Hanania, MD, FCCP (principal investigator), Marianna Sockrider, MD, DrPH (co-principal investigator), Laura Bertrand, RN, RPFT (principal clinic coordinator), Mustafa Atik, MD, Luz Giraldo, RCP, RCPT, Blanca A Lopez, LVN (coordinators); Columbia University-New York University Consortium: Joan Reibman, MD (principal investigator), Emily DiMango, MD, Linda Rogers, MD (co-principal investigators), Elizabeth Fiorino, MD (co-investigator at New York University), Cherie Cammarata, MSN, MA, Karen Carapetyan, MA (clinic coordinators at New York University), Jennifer Sormillon, BSN, Erin Simpson (clinic coordinators at Columbia University); Duke University Medical Center: Larry Williams, MD (principal investigator), John Sundy, MD, PhD (co-principal investigator), Peter Michelson, MD, MSC, Brian Vickery, MD, Ginny Dudek, RN (principal clinic coordinator), Rebecca Newton, RN, Amy Dug-dale, RN, Catherine Foss, BS, RRT, CCRC, Denise Jaggers, RN (coordinators); Emory University School of Medicine: W Gerald Teague, MD (principal investigator), Anne Fitzpatrick, PhD, RN, CPNP, Sumita Khatri, MD (co-principal investigators), Rachna Patel, BS (principal clinic coordinator), Jeannie Peabody, RN, Eric Hunter, BS, Denise Whitlock, RRT, CRP (coordinators); Illinois Consortium: Lewis Smith, MD (principal investigator), James Moy, MD, Adrienne Prestridge, MD (co-principal investigators), Jenny Hixon, BS, CCRC (principal clinic coordinator), Abbi Brees, BA, CCRC, Janine Judge, RRT (coordinators); Indiana University, Asthma Clinical Research Center: Michael Busk, MD, MPH (principal investigator), Paula Puntenney, RN, MA (principal clinic coordinator), Nancy Busk, BS, MS, Janet Hutchins, BSN (coordinators); University of Pennsylvania: Frank Leone, MD, MS (principal investigator), Michelle Hayes-Hampton, RN (principal clinic coordinator); National Jewish Health: Rohit Katial, MD (principal investigator), Marzena Krawiecz, MD (co-principal investigator), Holly Currier, RN (principal clinic coordinator); Nemours Children’s Clinic-University of Florida Consortium: John Lima, PharmD (principal investigator), Kathryn Blake, PharmD (co-principal investigator), Jason Lang, MD (co-principal investigator), David Schaeffer, MD (investigator), Donald George, MD (investigator), Mary Warde, RN, BSN (principal coordinator), Nancy Archer, RN, BSN (coordinator), Melissa McRae, RN (coordinator), Amber Santos, RN, MBA (coordinator); Hofstra University School of Medicine (formerly North Shore-Long Is-land Jewish Health System): Rubin Cohen, MD (principal investigator), Maria Santiago, MD (co-principal investigator), Ramona Ramdeo, MSN, FNP-C, RN, RT (principal clinic coordinator); Northern New England Consortium (formerly Vermont Lung Center at the University of Vermont): Charles Irvin, PhD (principal investigator), Anne E Dixon, MD, David A Kaminsky, MD, Thomas Lahiri, MD, Paul S Shapero, MD, (co-principal investigators), Richard Colletti, MD (gastro-intestinal consultant), Stephanie M Burns, Laurianne V Griffes, Roberta Pratt, RN, Michelle Doucette, RN, Pam Oertel (coordinators); Ohio State University Medical Center/Columbus Children’s Hospital: John Mastronarde, MD (principal investigator), Karen McCoy, MD (co-principal investigator), Jonathan Parsons, MD (co-investigator), Janice Drake, CCRC (principal clinic coordinator), Rachael A Compton, Laura Raterman, RN, David Cosmar, BA (coordinators); Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College: Allen J Dozor, MD (principal investigator), Sankaran Krishnan, MD, Jay Boyer MD, Nadav Traeger, MD (co-investigators), Ingrid Gherson, MPH (principal clinic coordinator), Lisa Monchil, RRT, CCRC (research coordinator); University of Alabama at Birmingham: Lynn B Gerald, PhD, MSPH (principal investigator), William C Bailey, MD, Roni Grad, MD (co-principal investigators), Sue Erwin, CRT (principal clinic coordinator), Ann Kelley, RN, Debra Laken, MaED, RRT, AE-C (coordinators); University of Miami, Miami University of South Florida: Adam Wanner, MD (principal investigator, Miami), Richard Lockey, MD (principal investigator, Tampa), Eliana S Mendes, MD (principal clinic coordinator for University of Miami), Shirley McCullough, BS (principal clinic coordinator for University of South Florida), Michelle Grandstaff-Singleton, BHSc, LPN, Diana Miller, BA (coordinators); University of Minnesota: Malcolm N Blumenthal, MD (principal investigator), Gail Brottman, MD, John Hagen, MD (co-principal investigators), Athena Decker, MA, Debra Lascewski, RN, Sheila Kelleher, MA (principal clinic coordinators), Kay A Bach-man, RN, Cindy Quintard, CCRC, Cherylee Sherry, MPH, CHES (coordinators); University of Missouri, Kansas City School of Medicine: Gary Salzman, MD (principal investigator), Chitra Dinakar, MD, Dennis Pyszczynski, MD (co-investigators), Patti Haney, RN, BSN, CCRC (principal clinic coordinator); St Louis Asthma Clinical Research Center, Washington University: Mario Castro, MD, MPH (principal investigator), Leonard Bacharier, MD, Kaharu Sumino, MD (co-investigators), Jaime J Tarsi, RN, MPH (principal coordinator), Brenda Patterson, MSN, RN, FNP (coordinator); University of California San Diego: Stephen Wasserman, MD (principal investigator), Joe Ramsdell, MD (co-principal investigator), Paul Ferguson, MS, Katie Kinninger, AS, Tonya Greene, AA (clinic coordinators); Chairman’s Office, University of Alabama, Birmingham (formerly at Respiratory Hospital, Winnipeg, Manitoba, Canada): William C Bai-ley, MD, Nicholas Anthonisen, MD, PhD (research group chair); Co-Principal Investigators’ Office, University of Virginia School of Medicine, Department of Pediatrics (formerly at Emory University): W Gerald Teague, MD (study co-principal investigator); Data Coordinating Center, Johns Hopkins University Center for Clinical Trials: Robert Wise, MD (centre director), Janet Holbrook, PhD, MPH (deputy director), Ellen Brown, MS (principal coordinator), Debra Amend-Libercci, Katherine Barry, BA, Marie Daniel, BA, Andrea Lears, BS, Gwen Leatherman, BSN, MS, RN, Charlene Levine, BS, Deborah Nowakowski, Nancy Prusakowski, MS, Sobharani Rayapudi, MD, ScM, Suzanna Roettger, MA, April Thurman, David Shade, JD, Elizabeth Sugar, PhD, Johnson Ukken, BA, Christine Wei, MS; Esophageal pH Probe Quality Control Center, Children’s Center for Digestive Healthcare Pediatric Gastroenterology, Hepatology, and Nutrition (formerly at Emory University School of Medicine): Benjamin Gold, MD (centre director); Data and Safety Monitoring Board: Stephen C Lazarus, MD (chair), William J Calhoun, MD, Michelle Cloutier, MD, Peter Kahrilas, MD, Bennie McWilliams, MD, Andre Rogatko, PhD, Christine Sorkness, PharmD; Project Office, American Lung Association: Elizabeth Lancet, MPH (project officer), Norman Edelman, MD (scientific consultant), Susan Rappaport, MPH; Project Office, National Heart, Lung, and Blood Institute: Virginia Taggart, MPH, Robert Smith, PhD (project officers), Gail Weinmann, MD (data and safety monitoring board executive secretary; deputy director, Division of Lung Diseases); American Lung Association Scientific Advisory Committee: E Neil Schacter, MD (chair), William C Bailey, MD, Mario Castro, MD, MPH, Brian Christman, MD, Amy Chuang, MD, Candy Holloway, Judith A Neubauer, PhD, Jonathan M Samet, MD, Erik Swenson, MD, Dona J Upson, MD, Daniel J Weiss, MD, PhD, Robert Wise, MD.
Funding
This work is funded by The American Lung Association (ALA) and The National Heart Lung and Blood Institutes (NHLBI).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception of this analysis, participated in the analysis, drafting and revision of the manuscript and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bime, C., Gerald, J., Wei, C. et al. Measurement characteristics of the childhood Asthma-Control Test and a shortened, child-only version. npj Prim Care Resp Med 26, 16075 (2016). https://doi.org/10.1038/npjpcrm.2016.75
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/npjpcrm.2016.75
This article is cited by
-
A systematic review of questionnaires measuring asthma control in children in a primary care population
npj Primary Care Respiratory Medicine (2023)
-
Effect of the Buteyko breathing technique on asthma severity control among school age children
The Egyptian Journal of Bronchology (2022)
-
Content validity of a newly developed observer-reported measure for pediatric asthma in children aged 2–5 years
Journal of Patient-Reported Outcomes (2022)
-
Development and equivalence of new faces for inclusion in the Childhood Asthma Control Test (C-ACT) response scale
Journal of Patient-Reported Outcomes (2021)