Abstract
Suitable tools for assessing the severity of chronic obstructive pulmonary disease (COPD) include multi-component indices and the global initiative for chronic obstructive lung disease (GOLD) categories. The aim of this study was to evaluate the dyspnoea, obstruction, smoking, exacerbation (DOSE) and the age, dyspnoea, obstruction (ADO) indices and GOLD categories as measures of current health status and future outcomes in COPD patients. This was an observational cohort study comprising 5,114 primary care COPD patients across three databases from UK, Sweden and Holland. The associations of DOSE and ADO indices with (i) health status using the Clinical COPD Questionnaire (CCQ) and St George’s Respiratory Questionnaire (SGRQ) and COPD Assessment test (CAT) and with (ii) current and future exacerbations, admissions and mortality were assessed in GOLD categories and DOSE and ADO indices. DOSE and ADO indices were significant predictors of future exacerbations: incident rate ratio was 1.52 (95% confidence intervals 1.46–1.57) for DOSE, 1.16 (1.12–1.20) for ADO index and 1.50 (1.33–1.68) and 1.23 (1.10–1.39), respectively, for hospitalisations. Negative binomial regression showed that the DOSE index was a better predictor of future admissions than were its component items. The hazard ratios for mortality were generally higher for ADO index groups than for DOSE index groups. The GOLD categories produced widely differing assessments for future exacerbation risk or for hospitalisation depending on the methods used to calculate them. None of the assessment systems were excellent at predicting future risk in COPD; the DOSE index appears better than the ADO index for predicting many outcomes, but not mortality. The GOLD categories predict future risk inconsistently. The DOSE index and the GOLD categories using exacerbation frequency may be used to identify those at high risk for exacerbations and admissions.
Similar content being viewed by others
Introduction
COPD is a growing burden on patients and healthcare systems, with a minority of patients being responsible for the majority of healthcare costs.1 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends that measurement of COPD severity should move from lung function alone to assessing current symptoms and future risk for exacerbations.2 Although current symptoms may be assessed using validated measures, identifying those at increased risk of exacerbations and admissions is more difficult. However, once identified, proven measures to minimise such risks may be applied to reduce the burden of exacerbations. These measures include optimal drug therapy, education for self-management and behavioural change such as smoking cessation, exercise and nutrition.2
A range of indices are available for use in routine clinical settings and none seem to be perfect for assessing prognosis.3 The value of a COPD severity index would be greatest if it (i) used items that are easy to collect, (ii) used items that are valid in their own right, (iii) provided better associations with patient-centred outcomes and healthcare consumption than with individual components and (iv) identified patients at increased risk for future events including exacerbations, hospital admissions and mortality.4
The GOLD strategy referred to three multi-component indices that assess COPD severity, the BODE, DOSE and ADO indices, but preferred a new system of GOLD categories based on current symptoms and future risk.2,5 The three multi-component indices were statistically derived and validated, whereas the GOLD categories were designed by an expert committee without statistical derivation. Subsequent studies have found that the GOLD categories do not accurately predict future risk.6–8
The BODE and ADO indices were derived as prognostic markers, but they also predict other patient-related outcomes including health status and functional capacity.9,10 However, the BODE index requires the 6-min walking test (6MWT), which limits its clinical use. The DOSE index was derived as a predictor of health status,11 but also reflects mortality,11,12 future exacerbations13 and decline in health status.14 The items used in these assessment systems are shown in Table 1.
The GOLD strategy produces four categories, based on current symptoms and future risk, which may be used to guide treatment. Several alternative methods are described to calculate the categories: future risk may either be estimated by the forced expiratory volume in one second as a percentage of predicted (FEV1%) or exacerbation frequency. Symptoms may be estimated using either the MRC dyspnoea scale, the COPD Assessment Test (CAT) or the Clinical COPD Questionnaire (CCQ).
In this study, we aimed to assess the value of the new GOLD categories and contrast them with existing multi-component indices suitable for use in primary care.
Results
Health status
The PRAXIS study consisted of 562 patients with 27% in GOLD airflow obstruction stage I, 41% in stage II, 25% in stage III and 7% in stage IV. The mean age was 63.6 years (standard deviation (s.d.) 7.7). During the average study follow-up period of five years, 116 patients (21%) died. The DOSE index ranged from 0 to 7 and 18% had an index of >4. The ADO index ranged from 0 to 8 and 39% had an index >4. In the Bocholtz data set the DOSE index ranged from 0 to 6 and 9% had an index >4. The ADO index ranged from 0 to 10 and 37% had an index >4. The Bocholtz and PRAXIS study data sets both showed that the DOSE index was more strongly correlated to various health status measures than the ADO index in cross-sectional data (Table 2).
Healthcare consumption
In the Optimum Patient Care (OPCRD) data set of 4,400 patients, the DOSE index ranged from 0 (mild) to 8 (severe); 24.5% (n=1,077) of subjects had a score of 4 or more. The ADO index ranged from 0 to 10 and 56.7% had an index >4. The prevalence of GOLD categories is shown in Figure 1. Although neither the DOSE nor the ADO index was a strong predictor of future events, the DOSE index performed better than its component items or the ADO index. The DOSE index was more highly correlated with number of exacerbations in the subsequent 12 months (r=0.32) compared with the ADO index (r=0.11, both P<0.001) to FEV1% (r=−0.06) and the MRC dyspnoea scale r=0.03). For a unit increase in DOSE or ADO index, the unadjusted odds ratio (95% confidence interval (CI)) for future exacerbation was 1.60 (1.52–1.67) and 1.14 (1.10–1.19), respectively. Similar figures for odds of future hospitalisation were DOSE 1.45 (1.27–1.65) and ADO 1.22 (1.07–1.39).
Table 3 shows the negative binomial regression models predicting the number of exacerbations and hospitalisations for DOSE and ADO indices and a range of healthcare measures.
Compared with models for the ADO index and those for healthcare measures such as FEV1%, smoking status, age or MRC scale, the model with DOSE as a predictor showed a better overall fit (as assessed by the lowest AIC). A similar picture was seen using number of exacerbations as the outcome. The unadjusted incident rate ratio for hospital admission in the subsequent year for patients with a DOSE index >4 was 2.97 (95% CI 2.03–4.36) and that for having an exacerbation in the next year was 2.99 (95% CI 2.66–3.37).
For the 7,105 OPCRD patients having both valid DOSE and ADO indices, receiver operator characteristic again showed that the DOSE index was a weak, but better predictor than the ADO index for future events. For hospitalisations, the areas under the curve for DOSE index was 0.64 (95% CI 0.57–0.71) and that for the ADO index was 0.57 (95% CI 0.50–0.64); for exacerbations, the areas under the curve for DOSE index was 0.58 (95% CI 0.55–0.60) and that for the ADO index was 0.53 (95% CI 0.50–0.55).
Mortality
Both DOSE and ADO index groups (score 4–5 or >5) were significantly associated with mortality (Table 4). Adjustment for gender and co-morbid cardiovascular disease did not substantially change the hazard ratios.
GOLD categories
Of the 3,015 patients in the OPCRD data set who had valid data on all the required items, the mean (s.d.) age was 72.6 (9.3) years. There were 342 (11.3%) non-smokers, 1,961 (65%) ex-smokers, 648 (21.5%) current smokers and 64 (2.1%) with missing smoking data.
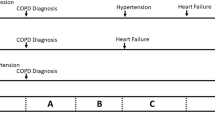
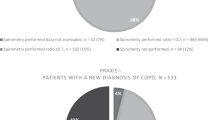
We compared the proportion of OPCRD patients across categories A to D when using alternative methods allowed in the GOLD strategy (Figure 1). The proportions varied greatly according to whether the MRC dyspnoea scale or CAT score was used for current symptoms or which of three methods was used for future risk (number of exacerbations, FEV1%, or either the FEV1% or number of exacerbations, whichever gives the highest risk). For example, 42% of patients were categorised into the more symptoms group when using the MRC dyspnoea scale versus 77% when using CAT; there were 3.1% in the high-risk group when using exacerbation frequency compared with 30% when using FEV1% and 32% when using the highest risk from either FEV1% or exacerbations. The proportion in the most severe group D varied from 2.8% using CAT and the exacerbation rate to 46% when using MRC for symptoms and the highest of exacerbation rate or FEV1% for future risk.
The GOLD categories were designed to predict future risk for exacerbations. When examined in relation to the proportion with an exacerbation in the next 12 months in the OPCRD data set, different results were found if alternative scoring systems were used (Figure 2). The use of baseline exacerbation rate rather than FEV1% showed much better discrimination in detecting future exacerbations. Using the highest risk or FEV1% on its own made little difference to the proportion with 1 or more exacerbations in the next 12 months.
Discussion
Main findings
In this paper, we compared the performance of the DOSE and ADO indices as measures of current and future disease severity in various COPD community cohorts. We found that the DOSE index showed a stronger association than the ADO index with a range of health status and healthcare consumption outcomes. In assessing future risk, we found that the hazard ratios for mortality were higher for ADO index groups than for DOSE index groups. Conversely, the DOSE index was a better predictor of risk for future exacerbations and hospital admissions than the ADO index, but previous exacerbations were the best predictor of future exacerbations in this study as in other studies.15
We found that alternative methods used to calculate the new GOLD categories resulted in large differences in the distribution of patient numbers in each category. The categories produced by different methods cannot be considered equivalent. As measures of future risk, the GOLD categories calculated using exacerbation rate had much higher odds ratios (data not shown) than those produced by FEV1% or the highest risk of FEV1% or exacerbation rate.
Interpretation of findings in relation to previously published research
Health status
When assessing the severity of COPD, the impact of the disease on patients’ lives in terms of current symptoms and limitations is critical, and is espoused by the GOLD strategy2 and the National Institute of Clinical Excellence (NICE) guidelines.5 This study confirms that the DOSE index is superior to, or as good as, the ADO index in its association with a wide range of patient-centred outcomes, including health status and quality of life measured by SGRQ and CCQ. The DOSE index has previously been shown to be a predictor of decline in health status.14
Mortality
This is not the first study to examine the prognostic value of multi-component indices. In 144 patients in secondary care, Oga et al.16 found that the BODE index had a superior discriminatory value over the DOSE and ADO indices as a predictor of mortality. Oga et al. also noted that the peak oxygen uptake (VO2 max) was superior to all three indices as a predictor of mortality. This finding builds on the original derivation studies of the BODE index, which highlighted the importance of including exercise testing in predicting mortality.
The present study confirms previous findings that the ADO index showed higher hazard ratios for mortality compared with the DOSE index.16,17 However, the ADO index has a greater contribution from age than other components; the older the patient, the higher the ADO index. Thus, a patient with severe breathlessness and airflow obstruction at the age of 40 years has, by definition, a lower ADO index than a similar patient aged 90 years. Most clinicians would feel that younger patients with severe symptoms and airflow obstruction would be of greater concern.18
The new GOLD categories do not perform well in predicting mortality; in large Spanish primary care data sets the new categories have no better prognostic value than the old FEV1-based GOLD stages.19 Thus, for prognostic assessment either the BODE or the ADO index is preferred.17
Predicting exacerbations and admissions
Patients at increased risk for exacerbations need to be identified, as it is known that optimal treatment may reduce the exacerbation rate using drugs, smoking cessation and pulmonary rehabilitation.15,20 Frequent exacerbators are at risk of faster decline in their health status, lung function and death.21–23 Both the DOSE index and the ADO index were weak predictors of future exacerbations in this study, and a previous report demonstrated an association with the DOSE index only.13 This study found the DOSE index to be a better predictor of future exacerbations than FEV1 or MRC dyspnoea scale, but the previous exacerbation rate was the best predictor.
The cost of hospital admissions is of major economic importance, and identifying those at risk would allow pre-emptive action to reduce that risk.24 A DOSE index of 4 or more was associated with an almost threefold increased risk for hospital admission. The DOSE index was a better predictor of admissions than the ADO and MRC dyspnoea scale, previous exacerbations or FEV1%. The DOSE index C-statistic compares with other more recently produced scores such as the SCOPEX, which examined only patients in clinical trial and risk of exacerbation over six months and included items such as ‘more COPD maintenance medications prior to the trial’.25 There are many potential candidate items for risk prediction models, including co-morbidities, biomarkers and blood oeosinophilia,26–28 but their place in prediction models is not yet clear.
The GOLD categories
The GOLD strategy (2011, updated 2015) advocated using a new assessment system of categories A–D based on current symptoms and future risk.2 The calculation of the four GOLD categories is complex and may not be practicable in primary care. The distribution within large primary care data sets has been demonstrated, but only using a limited range of possible methods of calculation.29 Our study is the first to examine all possible ways to calculate the GOLD categories. As in previous studies we found that the distribution into GOLD categories is uneven and varies greatly according to the method used to calculate them.8,19 It shows that the predictive value of future risk varies greatly according to the methods used to derive the categories. The treatment guidance based on future risk includes recommending inhaled steroids if they are ‘higher risk’; we found a 10-fold variation in the proportion of patients recommended inhaled steroids: only 3.1% in the high-risk group when using exacerbation frequency, compared with 30% when using FEV1% and 32% when using the highest risk from either FEV1% or exacerbations. If GOLD categories are to be used in practice, then their method of calculation must be stated, such as the subcategories C1, C2 and C3 and D1, D2 and D3.8 Unfortunately using subcategories adds to the complexity of using the system in clinical practice.
We have shown that, in considering the incident rate ratios of future exacerbations, the prior exacerbation rate had a stronger association than the FEV1%. The use of FEV1% in calculating future risk is thus not supported by evidence. If the GOLD categories are reviewed, we recommend that consideration be made to removing FEV1% as a measure of future risk and to use only exacerbation frequency.
Strengths and limitations of this study
Our data sets come from routinely collected data and are subject to missing items. Therefore, a number of patients’ data could not be included in the current analyses. In the OPCRD data set, as some people had data for less than the specified 12 months, seasonal bias may have influenced exacerbation rates. In primary care, routinely collected data on spirometry are of variable quality.30 Although this means that there may be less accurate diagnoses than in efficacy studies, the data sets reflect people with a clinical diagnosis in real life and only those patients whose FEV1/FVC ratio was <0.7 were included in the current analyses.
Some data sets comprised a small number of patients, resulting in limited statistical power to detect associations. Thus, caution needs to be employed in generalising these findings.
Implications for future research, policy and practice
When assessing patients in clinical practice, whether for quality improvement audits or for reporting patient status in primary and secondary care, we would concur with the NICE guidelines that the BODE index is the index of choice when 6MWT data are available.5 When the 6MWT is not available, the DOSE index is preferable to the ADO index for assessing current symptoms and future risk of exacerbations and hospitalisations. The risk of mortality is better predicted by the ADO or BODE indices than by the GOLD categories and the DOSE index.17 Given that the GOLD categories when calculated by the alternative methods produced greatly varying populations and variable value in predicting risk, they cannot be recommended for routine care or treatment decisions. They could be very valuable if simplified to use only exacerbations to define risk and CAT or CCQ to define symptoms.
Conclusions
Multidimensional systems for assessing COPD severity were assessed in real-life data from several community settings and none were found to be excellent. We found the DOSE index to be more closely associated than the ADO index with health status, exercise capacity, current exacerbations and hospital admissions. The DOSE index was a stronger predictor of hospital admissions and exacerbations in the subsequent year than the airflow obstruction or the MRC dyspnoea scale. The ADO index showed higher hazard ratios for mortality compared with the DOSE index. The GOLD categories allow alternative methods to calculate them, but the different methods produce very different groups and perform inconsistently as measures of future risk. Use of exacerbation frequency in the calculation of the GOLD categories produced better discrimination than FEV1%, and this approach could be extremely helpful in clinical care. If the BODE index data are not available, the ADO index is a better predictor of mortality than DOSE, but the DOSE index is better correlated with measures of current symptoms and future risk for exacerbations and hospitalisations.
Materials and methods
In a historical observational study using three existing databases of routinely collected clinical data, we performed an assessment of the GOLD categories and ADO and DOSE indices as measures of current symptoms and future risk. The component items of the indices and GOLD categories are shown in Table 1.
We investigated the associations of both the DOSE and ADO indices with current symptoms, exacerbations and hospital admissions, as well as with future events of exacerbations, admissions and mortality. As the GOLD categories are nominal variables, not linear scales, it was not possible to directly compare them with the DOSE and ADO indices. We examined whether the GOLD categories were equivalent when produced using the various alternative methods available and to evaluate how well they reflected current symptoms and future risks in large data sets of COPD patients in community settings encompassing the full range of COPD severity.
Data sets
The UNLOCK Group of the International Primary Care Respiratory Group31 and the Respiratory Effectiveness Group (www.effectivenessevaluation.org) provided a range of suitable data sets. From these we included people with a clinical diagnosis whose FEV1/FVC ratio was <0.7. The data sources used were:
-
1
UK primary care data from the OPCRD database (http://www.optimumpatientcare.org/Html_Docs/OPCRD.html). Data were routinely collected from 131 primary care practices on COPD patients in primary care. When the GOLD categories in the OPCRD data set were examined, we found 7,105 COPD patients aged 40 years and over who had valid MRC, exacerbation and FEV1% predicted values, as well as outcome variables including hospital admissions for exacerbations of COPD and future exacerbations. There were 4,400 patients with complete follow-up data, 3,015 of whom had valid CAT and MRC scores and a valid FEV1%.
-
2
Data from a pulmonary community rehabilitation clinical trial in Bocholtz, Holland, provided 152 primary care COPD patients over a two-year period, including data from the Saint Georges Respiratory Questionnaire (SGRQ) and the Clinical COPD Questionnaire (CCQ). The study provided 152 patients at baseline,32 133 at 12 months and 91 patients at 24 months; some variables were not recorded in all patients and valid numbers are as stated in the text.
-
3
Data from the PRAXIS study in Sweden consisted of 562 randomly selected patients, 34–75 years old, with a diagnosis of COPD from primary care (57%) and secondary care (43%) with complete DOSE and ADO indices. Data were collected in 2005 by self-completion questionnaire and record review during 2000–2003. Mortality data from 2005 to 2010 were provided from the Swedish Board of Health and Welfare.12
Primary care systems in different countries vary, but in Holland, UK and Sweden there is similar availability of drugs, guidelines and similar referral systems to secondary care and pulmonary rehabilitation (albeit without universal access). The baseline characteristics of the three cohorts are shown in the online supplement.
Statistical methods
Spearman’s rank correlation was used to assess the association between each of the DOSE and ADO indices and the health status measures and markers of healthcare consumption in each of the three data sets. In the OPCRD data set, logistic regression analysis was used to calculate the unadjusted odds ratio (95% CI) for one or more hospital admissions and for one or more exacerbations in the subsequent year for a unit increase in the DOSE or ADO index. Receiver operator characteristic analyses and areas under the curve (which is the equivalent of the c-statistic in a logistic model) were produced for DOSE and ADO indices in relation to the prediction of future risk for exacerbations and admissions for the OPCRD data. Negative binomial regression models were used to give unadjusted incident rate ratios (95% CI) of number of exacerbations and hospitalisations during follow-up for each of DOSE and ADO indices and a range of health status measures in the OPCRD data set. The Akaike’s information criterion (AIC) of each model was compared; the model with the lowest AIC was deemed the ‘best’ fit. All statistical models were based on a complete case analysis.
Using the PRAXIS study data, Cox regression was used to calculate the hazard ratio (95% CI) of mortality during follow-up for different DOSE and ADO index groups before and after adjustment for gender and pre-existing cardiovascular disease.
Using the OPCRD data set, descriptive methods were used to compare features of the GOLD categories when derived using the recommended alternative methods. Logistic regression was used to examine whether the GOLD patient group was an independent predictor of future admissions or exacerbations. Finally, the Kruskall–Wallis test was used to examine the distribution of DOSE and ADO indices across GOLD patient groups.
Ethical approval
The OPCRD data set has been approved for research by the Trent Research Ethics Committee. This project is covered by ADEPT approval. In the Dutch Bocholtz clinical trial, the regional Medical Ethics Committee of the Atrium Medical Centre, Heerlen, approved the study protocol. All patients gave written informed consent. The Swedish study was approved by the Regional Ethical Review Board of Uppsala University, Uppsala, Sweden (Dnr 2004:M-445, Dnr 2010/090 and Dnr 2012/252). Written consent to use the information for future analysis was obtained for all participating patients in 2005.
References
Wagner, E. H., Glasgow, R. & Davis, C. Quality improvement in chronic illness care: a collaborative approach. Jt Comm. J. Qual. Improv. 27, 63–80 (2003).
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD (2015) Available at http://www.goldcopd.org/http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html Accessed 10 November 2015.
Dijk, W. D. et al. Multidimensional prognostic indices for use in COPD patient care. A systematic review. Respir. Res. 12, 151 (2011).
Chavannes, N. H., Jones, R. C., Postma, D. S. & Rennard, S. Using COPD multidimensional indices in routine clinical practice: DOSE meets all criteria. Prim. Care. Respir. J. 21, 245–246 (2012).
National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: management of adults with chronic obstructive pulmonary disease in primary and secondary care. NICE Guideline (CG101.) (2010).
Lange, P. et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am. J. Respir. Crit. Care Med. 186, 975–981 (2012).
de Torres, J. P. et al. Prognostic evaluation of COPD Patients: GOLD11 versus BODE and COPD comorbidity index COTE. Thorax 69, 799–804 (2014).
Han, M. K. et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med 1, 43–50 (2013).
Puhan, M. A. et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 374, 704–711 (2009).
Ong, K. C., Lu, S. J. & Soh, C. S. Does the multidimensional grading system (BODE) correspond to differences in health status of patients with COPD? Int. J. Chron. Obstruct. Pulmon. Dis. 1, 91–96 (2006).
Jones, R. C. et al. Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease—The DOSE Index. Am. J. Respir. Crit. Care Med. 180, 1189–1195 (2009).
Sundh, J. et al. The Dyspnoea, Obstruction, Smoking, Exacerbation (DOSE) index is predictive of mortality in COPD. Prim. Care Respir. J. 21, 295–301 (2012).
Motegi, T. J. et al. A comparison of three multidimensional indices of COPD severity as predictors of future exacerbations. Int. J. Chron. Obstruct. Pulmon. Dis. 8, 259–271 (2013).
Rolink MvD, W., van den Haak-Rongen, S., Pieters, W., Schermer, T. & van den Bemt, L. Using the DOSE index to predict changes in health status of chronic obstructive pulmonary disease patients. Prim. Care Respir. J. 22, 169–174 (2013).
Hurst, J. R. et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 363, 1128–1138 (2010).
Oga, T., Tsukino, M., Hajiro, T., Ikeda, A. & Nishimura, K. Predictive properties of different multidimensional staging systems in patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 6, 521–526 (2011).
Marin, J. M. et al. Multicomponent indices to predict survival in COPD: the COCOMICS study. Eur. Respir. J. 42, 323–332 (2013).
Celli, B. R., Marin, J. M., Cote, C. G., Aguirre, A. & Macario, C. C. Prognostic assessment of patients with COPD. Lancet 374, 1885 (2009).
Soriano, J. B. et al. Distribution and prognostic validity of the new global initiative for chronic obstructive lung disease grading classification. CHEST 143, 694–702 (2013).
Department of Health. An outcomes strategy for people with chronic obstructive pulmonary disease (COPD) and asthma in England. London (2011) Gateway reference 16249.
Donaldson, G. C., Seemungal, T. A., Bhowmik, A. & Wedzicha, J. A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 57, 847–852 (2002).
Seemungal, T. A. et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 157 (5 Pt 1): 1418–1422 (1998).
Soler-Cataluna, J. J. et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 60, 925–931 (2005).
Bryden, C., Bird, W., Titley, H. A., Halpin, D. M. & Levy, M. L. Stratification of COPD patients by previous admission for targeting of preventative care. Respir. Med. 103, 558–565 (2009).
Make, B. J. et al. A score to predict short-term risk of COPD exacerbations (SCOPEX). Int. J. Chron. Obstruct. Pulmon. Dis. 10, 201–209 (2015).
Taylor, D. R. Risk assessment in asthma and COPD: a potential role for biomarkers? Thorax 64, 261–264 (2009).
Pascoe, S., Locantore, N., Dransfield, M. T., Barnes, N. & Pavord, I. D. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir. Med. 3, 435–442 (2015).
Price, D. et al. Blood eosinophilia and the number of exacerbations in COPD patients. Eur. Respir. J. 44 (Suppl 58) (2014).
Haughney, J., Gruffydd-Jones, K., Roberts, J., Hardwell, A. & McGarvey, L. The distribution of COPD in UK general practice using the new GOLD classification. Eur. Respir. J. 43, 993–1002 (2014).
Jones, R. C., Dickson-Spillmann, M., Mather, M. J., Marks, D. & Shackell, B. S. Accuracy of diagnostic registers and management of chronic obstructive pulmonary disease: the Devon primary care audit. Respir. Res. 9, 62 (2008).
Chavannes, N. et al. UNLOCK: Uncovering and noting long-term outcomes in COPD to enhance knowledge. Prim. Care Respir. J 19, 408 (2010).
Chavannes, N. H. et al. Integrated disease management improves one-year quality of life in primary care COPD patients: a controlled clinical trial. Prim. Care Respir. J. 18, 171–176 (2009).
Acknowledgements
We thank Sian Williams of the International Primary Care Respiratory Group for her help and encouragement with the project. The OPCRD database was made available courtesy of the Respiratory Effectiveness Group and RIRL and the data were kindly prepared for analysis by Julie von Ziegenweidt.
Funding
The International Primary Care Respiratory Group (IPCRG) provided funding for this research project as an UNLOCK group study for which the funding was obtained through an unrestricted grant by Novartis AG, Basel, Switzerland. The latter funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Database access for the OPCRD was provided by the Respiratory Effectiveness Group (REG) and Research in Real Life; the OPCRD statistical analysis was funded by REG. The Bocholtz Study was funded by PICASSO for COPD, an initiative of Boehringer Ingelheim, Pfizer and the Caphri Research Institute, Maastricht University, The Netherlands.
Author information
Authors and Affiliations
Consortia
Contributions
The project was conceived and conducted as an UNLOCK study of the IPCRG and was run in conjunction with the Respiratory Effectiveness Group. RJ, DP, NC, BS, MH, Kl, TvdM and IT were all involved in the concept and design of the study, AL and JS conducted the analyses, and all authors contributed in writing the paper.
Corresponding author
Ethics declarations
Competing interests
NHC, BS and IT are Associate Editors of npj Primary Care Respiratory Medicine, but were not involved in the editorial review of, nor the decision to publish, this article. RCJ reports personal fees from Almirall, Astra Zeneca, Boehringher Ingelheim, Chiesi, Cipla, GSK; grants, personal fees, non-financial support from Novartis; personal fees and non-financial support from Napp/Mundi Pharma. DP has board membership with Aerocrine, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis and Teva Pharmaceuticals; consultancy with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis and Teva Pharmaceuticals; grants and unrestricted funding for investigator-initiated studies from UK National Health Service, British Lung Foundation, Aerocrine, AKL, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline, Meda, Merck, Mundipharma, Napp, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva Pharmaceuticals and Zentiva; payments for lectures/speaking from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, SkyePharma, Takeda and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; patents (planned, pending or issued) from AKL; payment for the development of educational materials from GlaxoSmithKline and Novartis; stock/stock options from AKL which produces phytopharmaceuticals; owns 80% of Research in Real Life and its subsidiary social enterprise Optimum Patient Care; received payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis and Teva Pharmaceuticals; funding for patient enrolment or completion of research from Almirral, Chiesi, Teva Pharmaceuticals and Zentiva; and peer reviewer for grant committees of the Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012), HTA (2014). AJL received fees from the Respiratory Effectiveness Group and from PUPSMD during the conduct of the study. BS has received honoraria for educational activities and lectures from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Meda, MSD, Novartis and TEVA, and has served on advisory boards arranged by AstraZeneca, Novartis, GlaxoSmithKline and Boehringer. Ingelheim K Lisspers has received payments for educational activities and lectures from AstraZeneca, GlaxoSmithKline, Meda, MSD, Novartis and Nycomed and has served on advisory boards arranged by Novartis. TvdM reports Advisory Board Membership: Almiral, Astra Zeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma, Novartis, and Teva. Consultancy: Almiral, Astra Zeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, MSD, Mundipharma, Novartis, Nycomed, Certe and Teva. His research department at the University of Groningen has received grants and support for research in respiratory disease from the following organisations in the last 5 years: the lung foundation Netherlands, Stichting bestrijding Astma, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Mundipharma, Novartis, Nycomed. Payments for lectures/speaking: Almiral, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Merck, Mundipharma,and Teva Patents (planned, pending or issued): CCQ copyrights ICQ copyrights BHQ copyrights. IT has received payments for participation in advisory boards and lectures from Boehringer Ingelheim and Novartis. The remaining authors declare no conflict of interest.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Jones, R., Price, D., Chavannes, N. et al. Multi-component assessment of chronic obstructive pulmonary disease: an evaluation of the ADO and DOSE indices and the global obstructive lung disease categories in international primary care data sets. npj Prim Care Resp Med 26, 16010 (2016). https://doi.org/10.1038/npjpcrm.2016.10
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/npjpcrm.2016.10
This article is cited by
-
The prevalence of comorbidities in COPD patients, and their impact on health status and COPD symptoms in primary care patients: a protocol for an UNLOCK study from the IPCRG
npj Primary Care Respiratory Medicine (2016)