Abstract
Background:
When standard doses of inhaled corticosteroids (ICS) fail to control symptoms in children aged >4 years, guidelines recommend the addition of a long-acting β2-agonist (LABA), with other treatment options being available if symptoms persist.
Aims:
To determine the proportion of initial ‘step-up’ episodes where LABAs were prescribed and to describe characteristics of individuals not stepped up with LABA.
Methods:
Between 1999 and 2011, initial step-up episodes from ICS monotherapy were identified in children aged 5–12 years with asthma and in receipt of ICS. Data sources were the Clinical Practice Research Datalink and Optimum Patient Care Research Database.
Results:
Initial step-up episodes were identified in 10,793 children. ICS dose was increased in 6,252 children (58%), LABA was introduced in 3,436 (32%; including 1,107 where fixed dose combination inhaler (FDC) replaced the ICS inhaler), and leukotriene receptor antagonist (LTRA) was added in 1,105 (10%). Compared with children stepped up to any LABA, others were younger and prescribed lower doses of ICS and reliever medication. ICS dose increase was more likely in obese children and LTRA prescribing was more likely in children with rhinitis and in receipt of antibiotics. Compared with FDC, step-up to separate LABA inhaler was more likely in younger, obese children who were using less oral steroids.
Conclusions:
One-third of initial step-up episodes in children with asthma treated with ICS are to add LABA. Different characteristics of children prescribed therapies other than LABA suggest that prescribers tailor treatment in some clinical settings.
Similar content being viewed by others
Introduction
Asthma is a common condition1 and 5% of children in the UK are prescribed inhaled corticosteroids (ICS) to control asthma symptoms.2 Annually there are over 25,000 children admitted to hospital for asthma in England and Wales3 and the cost of asthma inhaler prescriptions issued in the UK to children aged 5–15 years is ~£37million,4 which is equivalent to £5 for every child in the population. Treatment with low dose ICS is effective in controlling symptoms for the majority of children but ~10% of children require one of four additional ‘step-up’ treatment options.1,2 Clinical trials have demonstrated that many children will benefit from more than one step-up option5–10 but equally, some will gain greatest benefit from one of the options.5,10
In the absence of evidence for a ‘best’ step-up option, guidelines for asthma management in children advocate either addition of LABA1,11 or increasing ICS dose12 as the first step-up option while highlighting the need to assess response and prescribe an alternative treatment for non-responders. More recent guidance recommends that step-up to fixed dose combination inhaler (FDC) containing ICS and LABA is preferable to addition of separate LABA inhaler due to better adherence to a single inhaler device.13 What is not known is whether these guidelines1,13 have been put into practice and our main research question was ‘in what proportion of first step-up episodes is LABA treatment added to ICS rather than ICS dose increased or LTRA added?’. Given the uncertainty as to which initial treatment step-up is most effective, we anticipated that some children would be stepped up to treatment other than LABA and tested the null hypothesis that children stepped up to LTRA or increased ICS would be no different to those stepped up to LABA; the presence of differences between those stepped up to LABA and to other medications would suggest that prescribers recognise subgroups who they feel are more likely to respond to options other than LABA. We tested our research question and hypothesis using routinely acquired ‘real life’ data from primary care, an approach which complements results from clinical trials14 and has been used in the paediatric setting15 and also, in adults, in the context of stepping up treatment.16
Materials and methods
Study population
Prescribing data were obtained from the CPRD and the OPCRD between 1 January 1990 and 23 December 2011 for OPCRD and 11 April 2011 for CPRD. Data before 1 January 1999 were not included since LTRA and FDC inhalers were not licensed for use in the UK until 1998 and 1999, respectively. Inclusion criteria were (i) asthma diagnosis, defined as a Read code for asthma and/or >1 prescription for asthma medications (including ⩾1 for ICS) in the 12 months prior to the first step-up date (index date), (ii) aged 5–12 years when treatment stepped up to allow comparison with recommendations for this age range set out in the BTS/SIGN guideline,1 (iii) registered with the practice for at least 12 months prior to and post index date. Exclusion criteria were (i) other chronic respiratory disease, (ii) maintenance oral steroid prescribed in the 12 months prior to the index date, (iii) previous prescription for LABA (as separate or FDC inhaler), LTRA or theophylline in the year prior to the index date, (iv) multiple step-up therapies, multiple ICS, or prescription of both FDC and ICS inhalers at index date, (v) ICS step-up dose by <50%,17 and (vii) prescribed theophylline at step-up (due to small numbers).
Study design
Patients were categorized according to their first step-up as: (i) addition of LABA, ICS dose unchanged, (ii) increase ICS dose, (iii) addition of LTRA, ICS dose unchanged, and (iv) replacement of ICS inhaler with FDC. Distinction was made between FDC and addition of separate LABA inhaler since the former is the recommended option.13 This study was approved in 2010 by the Independent Scientific Advisory Committee of the (then) General Practice Research Database.
Predictor variables (or patient characteristics)
Patient characteristics and asthma medication use were defined over the 12 months prior to step-up. Rhinitis diagnosis was ‘rhinitis ever’ as coded in primary care (Read) records. Eczema medications were those prescribed in the 12 months prior to step-up. SABA use was defined as any prescription in the baseline year. Body mass index (BMI) was derived from height and weight and converted to centiles and z-scores with reference to the 1990 UK standard18 applying clinical cutoffs for overweight (⩾91st centile) and obese (⩾98th centile). BMI z-scores outside the range ±5 were excluded as presumed data entry mistake.19 Paracetamol prescription in the previous year was included since this is a confounder in adult studies.20 Ethnicity data were not available. The online supplement describes the methods for deriving the following variables: average daily ICS dosage, average daily short-acting β2-agonist (SABA) use, acute oral steroid use, and medication possession ratio.
Clinical practice research datalink
The Clinical practice research datalink (CPRD), initially the General Practice Research Database, was established in 1990 and by 1999 included a stable population of 650 practices across the UK and provided primary care prescribing for ~5% of the population. (http://www.cprd.com/intro.asp).
Optimum patient care research database
The Optimum Patient Care Research Database (OPCRD) was established in 2008 and currently has 424 practices in the database. Patients with respiratory conditions are actively recruited. The OPCRD holds data from 1,200,014 patients including 514,719 with asthma; therefore OPCRD has data on ~10% of the UK asthma population. Algorithms identify any patients included from both CPRD and OPCRD and remove duplicates. (http://www.optimumpatientcare.org/Html_Docs/OPCRD.html).
Statistical analysis
Differences across the categories of ICS dose increases and also the univariate associations between first post ICS step-up therapy and each patient characteristic or baseline asthma medication variable were tested using a Χ2-test for categorical variables and Kruskal–Wallis for variables measured on the interval or ratio scale. A multivariate regression model was built using multinomial logistic regression with FDC as the reference group, by first including age, sex, index year, and all variables with univariate associations with P<0.1 in the model. Variables were examined for co-linearity and clinical importance then removed in a backwards stepwise procedure until all variables remaining in the multivariate model had P<0.1. Since addition of LABA has until recently been the recommended first step-up option, we repeated analyses with this option as the reference. All analyses were carried out using SAS version 9.3 (SAS, Cary, NC, USA).
Results
Study subjects
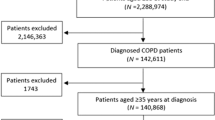
Data were available in 10,793 individuals (7,609 from OPCRD) whose median (interquartile range) age was 9 (6–11) years, 59% were male, 97% had a diagnosis of asthma, and 50% were prescribed eczema medications (Table 1). Figure 1 shows how individuals were identified from the databases. There were 6,252 children where ICS dose was increased (58%), 2,329 were prescribed a separate LABA inhaler (22%), 1,107 switched to FDC (10%), and 1,105 had LTRA added (10%). Table 2 describes the characteristics of individuals where ICS dose was increased stratified by dose increase option. These seven groups included 96% of all ICS dose step-up episodes and differed by age and year when step-up was made. The most common ICS dose increase was from 200 to 400 µg (budesonide equivalent) which was prescribed in 4,135 children (66% of the ICS dose increase group). Supplementary Table E1 presents the number of children in each of the possible 154 ICS step-up dose categories.
Changes over time and univariate differences between groups
The incidence of ICS step-up fell between 1999 and 2005, coinciding with a rise in overall LABA prescribing (that is, FDC plus separate LABA inhaler), but remained static between 2005 and 2011 (Figure 2). The incidence of all LABA prescribing fell and LTRA prescribing rose between 2005 and 2011 (Figure 2 and online Supplementary Table E2). In univariate analyses, the following characteristics at baseline differed across the four treatment groups: age, year when step-up was made, obesity, eczema, daily dose of ICS, medication possession ratio, need for SABA use, oral corticosteroid, and antibiotics in the previous year, primary care consultations, and data source (OPCRD versus CPRD) (Table 3).
Proportion of step-up options over time in children with asthma already in receipt of inhaled corticosteroid treatment (ICS). All LABA, FDC plus LABA; FDC, fixed dose combination inhaler; LABA, addition of long-acting β2-agonist as separate inhaler; LTRA, leukotriene receptor antagonist. Data from 2010 and 2011 are combined since data from the whole of the calendar year 2011 were not available from CPRD. The vertical broken lines indicate when British Thoracic Society/Scottish Intercollegiate Guidelines network (BTS/SIGN) asthma guidelines were published.
Multivariate analysis
In the multivariate analysis and in comparison with all other groups, children in the FDC group were older and more likely to have seen the GP for asthma (Table 4). The FDC group was more likely to have received oral steroid treatment but less likely to have received antibiotics compared with the LABA and LTRA groups, less likely to be obese and more likely to have regular use of SABA compared with the ICS dose increase group, and more likely to have received an average daily dose of ICS >200 µg (BDP equivalent) than ICS and LTRA groups (Table 4). Children in the LTRA group were more likely to have rhinitis and to have received more than one antibiotic for respiratory symptoms in the previous year (Table 4). Supplementary Table E3 compares the characteristics of children in treatment groups with reference to addition of a separate LABA inhaler.
Discussion
Main findings
This is the first study to describe which initial ‘step-up’ asthma treatment options are made for 5–12-year-old children in the UK, and also the first to describe the characteristics of children who receive different step-up options. The first main finding was that step-up with increasing ICS dose remained the most popular step-up option between 1999 and 2011 despite national guidelines recommending step-up with LABA before considering increasing ICS dose to 400 µg BUD equivalent per day.1 The second novel finding was that children who were stepped up to medication other than LABA had a number of characteristics which were subtly different to those where LABA was introduced (Figure 3), and this suggests that clinicians may be making active decisions not to add LABA. Our results are based on observations from ~15% of children in England and are therefore likely to be generalisable. The hypothesis that children with some characteristics, for example, obesity and rhinitis, gain greater benefit from treatment other than LABA needs to be tested in large populations.
Strengths and limitations of this study
There are a number of strengths and limitations to this study. Strengths include the large number of individuals included, adjustment for health seeking behaviour, and collection of prescriptions and the use of routine data, which avoids the bias in recruitment which can occur in clinical trials making generalisation unreliable.14 A limitation of routinely entered data is that data are incomplete leading to some individuals being excluded. A second limitation to our study is that data were not complete for all individuals studied, for example, BMI was available for 65% of children, and while missing values might not be at random we have demonstrated that children with missing BMI data were equally distributed across step-up groups. A final limitation is that in an observational study such as ours, causation cannot necessarily be inferred from the associations described.
Interpretation of findings in relation to previously published work
The majority of initial step-up episodes were to increase ICS dose, and over 70% of these were increases to a daily dose of 400 µg budesonide equivalent. Approximately 10% of ICS step-ups were from a daily dose of 100 to 200 µg budesonide, and although these occurred in a younger group of individuals, suggesting that clinicians were being more cautious with ICS dose in younger children, it is possible that some incidents represented ‘fine tuning’ doses from an initially very low value to the dose suggested for step 2 treatment.1 Notwithstanding this small number of step-ups to 200 µg, the majority of ICS step-ups were consistent with a move to the BTS/SIGN step 3 treatment step but these episodes were not preceded with LABA treatment and this is not consistent with the guideline.1
Table 2 demonstrates that the majority (69%) of ICS step-up options were from 200 to 400 µg budesonide per day, and this suggests that that clinicians looking after patients in the real world have found that stepping up from low to intermediate ICS dose is a safe and effective option and that this accounts for its relative popularity. Eight percent of ICS step-ups were either from low direct to high dose or to ICS dose in excess of the ceiling dose of 800 µg budesonide equivalent and these observations replicate our earlier work highlighting the prevalence of high dose ICS prescribing.2 The BTS/SIGN1 guideline suggests that children in receipt of high dose ICS (that is, 800 µg budesonide per day) should receive specific written advice about steroid replacement and be under the care of a specialist paediatrician.
Asthma prescribing is known to deviate from guidelines in the UK,21,22 and other countries,23,24 and might in part be due to a delay between guidelines being published and implemented; other reasons for prescribing changes include falls in pricing as medications come off patent and concerns about adverse effects of LABA therapy raised by the United States Food and Drug Administration in 2005.25 The BTS/SIGN guidelines first recommend LABA addition rather than increasing ICS dose since 1995,26 and this change in advice might explain the continuing rise in LABA prescribing between 1999 and 2005. The 2003 BTS/SIGN guideline was the first to recommend inclusion of LTRA27 but this advice was anticipated since 100,000 LTRA prescriptions for children were made in 200322 and we see rising LTRA prescribing in the early 2000s. What is notable is that despite LABA being advocated as the first step by guidelines since 1995, the rise in LABA prescribing pre 2006 has faltered, as has been demonstrated previously2,22 and here we show a decline in LABA prescribing between 2006 and 2011; this study cannot explain why LABAs are less frequently prescribed but one explanation might be that prescribers find LTRA or increasing ICS dose to be more effective. An alternative explanation for increasing step-up by addition of LTRA or increasing ICS in younger children within the 5–12-year-old category may be ‘carry over’ of more recent BTS/SIGN1 and GINA12 guideline advice to use these step-up options in the <5-year-old category.
Implications for future research, policy and practice
The presence of differences between individuals in the step-up option groups suggests that prescribers perceive that some individuals gain benefit from specific step-up options. In a tertiary care setting, clinicians have previously been shown to prescribe asthma medications by phenotype, that is, viral wheeze versus multitrigger wheeze,28 with the implication that some individuals are recognised as having preferential response to some asthma medications. In one study, differential response to step-up therapy has been linked to age, the presence of eczema and ethnicity,5 while in a second study increased reliever medication use, increased exhaled nitric oxide, and reduced lung function were more likely following increasing ICS dose compared with the addition of LTRA.10 Obese children are less responsive to ICS therapy,29 but obese children were 40% more likely to have ICS step-up rather than addition of FDC in our study. We observed that children stepped up to LTRA had a higher prevalence of rhinitis and a 70% increased risk for multiple prescriptions for antibiotics for respiratory symptoms, when compared with both LABA step-up options, and this decision may be justifiable since LTRA are known to be effective against upper and lower respiratory tract symptoms.30 Our study raises at least two research questions which we will address in future work: (i) what are the outcomes of stepping up asthma treatment using FDC as the gold standard in comparison with increasing ICS or addition of LTRA or LABA separately?; (ii) are there patient subgroups (or phenotypes) who gain preferential response from options other than step-up to FDC?
Conclusions
Guidelines are one of the critical components of good clinical practice but are limited by their one-size-fits-all approach and, in the case of stepping up asthma treatment in children, often lack an evidence base. The clinical setting of a child whose asthma symptoms are not adequately controlled with ICS is common and there is a lack of evidence for ‘best practice’; in this context it is appropriate for clinicians to consider guideline advice but not necessarily apply this advice. Our results demonstrate that clinicians in the UK frequently do not adhere to the BTS/SIGN guideline for asthma,1 and deviation from recommendations appears to be an active decision in circumstances where a number of coexisting factors are present. An individualised approach may be more effective in guiding the treatment of asthma beyond step 2 (ICS therapy), which is based around an asthma action plan and in future guided by genetic factors31 or biomarkers such as exhaled nitric oxide.32
References
British Thoracic Society and Scottish Intercollegiate Guidelines Network. BTS/SIGN Guideline on Asthma Management 2012. https://www.brit-thoracic.org.uk/guidelines-and-quality-standards/asthma-guideline/. Accessed 29 August 2014.
Turner S, Thomas M, von Ziegenweidt J, Price D . Prescribing trends in asthma: a longitudinal observational study. Arch Dis Child 2009; 94: 16–22.
Millett C, Lee JT, Laverty AA, Glantz SA, Majeed A . Hospital admissions for childhood asthma after smoke-free legislation in England. Pediatrics 2013; 131: e495–e501.
National Institute for Clinical Excellence. Inhaler Devices for Routine Treatment of Chronic Asthma in Older Children (aged 5–15 years) 2002. http://www.nice.org.uk/guidance/ta38. Accessed 19 August 2014.
Lemanske RF Jr, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD et al. Childhood Asthma Research and Education (CARE) Network of the National Heart, Lung,and Blood Institute. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med 2010; 362: 975–985.
de Blic J, Ogorodova L, Klink R, Sidorenko I, Valiulis A, Hofman J et al. Salmeterol/fluticasone propionate vs. double dose fluticasone propionate on lung function and asthma control in children. Pediatr Allergy Immunol 2009; 20: 763–771.
Gappa M, Zachgo W, von Berg A, Kamin W, Stern-Strater C, Steinkamp G et al. Add-on salmeterol compared to double dose fluticasone in pediatric asthma: a double-blind, randomized trial (VIAPAED). Pediatr Pulmonol 2009; 44: 1132–1142.
Murray CS, Custovic A, Lowe LA, Aldington S, Williams M, Beasley R et al. Effect of addition of salmeterol versus doubling the dose of fluticasone propionate on specific airway resistance in children with asthma. Allergy Asthma Proc 2010; 31: 415–421.
Vaessen-Verberne AA, van den Berg NJ, van Nierop JC, Brackel HJ, Gerrits GP, Hop WC et al. Combination therapy salmeterol/fluticasone versus doubling dose of fluticasone in children with asthma. Am J Respir Crit Care Med 2010; 182: 1221–1227.
Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol 2005; 115: 233–242.
Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Gotz M et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy 2008; 63: 5–34.
Global Initiative for Asthma. Pocket Guide for Asthma Management and Prevention 2015. http://www.ginasthma.org/documents/1/Pocket-Guide-for-Asthma-Management-and-Prevention. Accessed 29 August 2014.
National Institute for Clinical Excellence. Inhaled Corticosteroids for the Treatment of Chronic Asthma in Children Under the Age of 12 years 2012. http://www.nice.org.uk/guidance/ta131. Accessed 29 August 2014.
Holgate S, Bisgaard H, Bjermer L, Haahtela T, Haughney J, Horne R et al. The Brussels Declaration: the need for change in asthma management. Eur Respir J 2008; 32: 1433–1442.
Wu AC, Li L, Fung V, Kharbanda EO, Larkin EK, Vollmer WM et al. Use of leukotriene receptor antagonists are associated with a similar risk of asthma exacerbations as inhaled corticosteroids. J Allergy Clin Immunol Pract 2014; 2: 607–613.
Thomas M, von Ziegenweidt J, Lee AJ, Price D . High-dose inhaled corticosteroids versus add-on long-acting beta-agonists in asthma: an observational study. J Allergy Clin Immunol 2009; 123: 116–121, e10.
Price D, Chisholm A, Hillyer EV, Burden A, vo Zeigenweidt J, Svadsater H et al. Effect of inhaled corticosteroid therapy step-down and dosing regimen on measures of asthma control. J Aller Ther 2011; 4: 126.
Cole TJ, Freeman JV, Preece MA . Body mass index reference curves for the UK, 1990. Arch Dis Child 1995; 73: 25–29.
Smith SM, Craig LC, Raja AE, McNeill G, Turner SW . Growing up before growing out: secular trends in height, weight and obesity in 5--6-year-old children born between 1970 and 2006. Arch Dis Child 2013; 98: 269–273.
Price D, Martin RJ, Barnes N, Dorinsky P, Israel E, Roche N et al. Prescribing practices and asthma control with hydrofluoroalkane-beclomethasone and fluticasone: a real-world observational study. J Allergy Clin Immunol 2010; 126: 511–518, e1-10.
Elkout H, Helms PJ, Simpson CR, McLay JS . Changes in primary care prescribing patterns for paediatric asthma: a prescribing database analysis. Arch Dis Child 2012; 97: 521–525.
Cohen S, Taitz J, Jaffe A . Paediatric prescribing of asthma drugs in the UK: are we sticking to the guideline? Arch Dis Child 2007; 92: 847–849.
Karlstad O, Nafstad P, Tverdal A, Skurtveit S, Furu K . Prevalence, incidence and persistence of anti-asthma medication use in 2- to 29-year-olds: a nationwide prescription study. Eur J Clin Pharmacol 2010; 66: 399–406.
Sen EF, Verhamme KM, Neubert A, Hsia Y, Murray M, Felisi M et al. Assessment of pediatric asthma drug use in three European countries; a TEDDY study. Eur J Pediatr 2011; 170: 81–92.
Martinez FD . Safety of long-acting beta-agonists--an urgent need to clear the air. N Engl J Med 2005; 353: 2637–2639.
The British Guidelines on Asthma Management 1995 Review and Position Statement. Thorax 1997; 52(Suppl 1): S1–S20.
Guidelines on the management of asthma. Statement by the British Thoracic Society, the Brit. Paediatric Association, the Research Unit of the Royal College of Physicians of London, the King's Fund Centre, the National Asthma Campaign, the Royal College of General Practitioners, the General Practitioners in Asthma Group, the Brit. Assoc. of Accident and Emergency Medicine, and the Brit. Paediatric Respiratory Group. Thorax 1993; 48(2 Suppl): S1–S24.
Ducharme FM, Morin J, Davis GM, Gingras J, Moya FJ . High physician adherence to phenotype-specific asthma guidelines, but large variability in phenotype assessment in children. Curr Med Res Opin 2012; 28: 1561–1570.
Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedon JC et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol 2011; 127: 741–749.
Roberts G, Xatzipsalti M, Borrego LM, Custovic A, Halken S, Hellings PW et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy 2013; 68: 1102–1116.
Lipworth BJ, Basu K, Donald HP, Tavendale R, Macgregor DF, Ogston SA et al. Tailored second-line therapy in asthmatic children with the Arg(16) genotype. Clin Sci 2013; 124: 521–528.
Mahr TA, Malka J, Spahn JD . Inflammometry in pediatric asthma: a review of fractional exhaled nitric oxide in clinical practice. Allergy Asthma Proc 2013; 34: 210–219.
Acknowledgements
The CPRD data was made available through an unrestricted grant to GPRD from the Medical Research Council. We are grateful to Research in Real Life for donating the OPCRD data. The CPRD data was made available through an unrestricted grant to GPRD from the Medical Research Council. We are grateful to Research in Real Life for donating the OPCRD data and the Respiratory Effectiveness Group for funding the analysis.
Funding
The Respiratory Effectiveness Group funded the analysis
Author information
Authors and Affiliations
Contributions
The study was conceived by CM, DP, MT, and ST. Data extraction and analysis were undertaken by KR and AB. The first draft was written by ST. All authors have made meaningful contributions to the submitted manuscript. ST is the guarantor for this work.
Corresponding author
Ethics declarations
Competing interests
MT: Neither MT nor any member of his close family has any shares in pharmaceutical companies. In the last 3 years he has received speaker’s honoraria for speaking at sponsored meetings or satellite symposia at conferences from the following companies marketing respiratory and allergy products: Aerocrine, Astra Zeneca, Boehringer Inglehiem, GSK, MSD, and Teva. He has received honoraria for attending advisory panels with; Aerocrine, Almirall, Astra Zeneca, BI, Chiesi, GSK, MSD, and Novartis. He has received sponsorship to attend international scientific meetings from: GSK, Astra Zeneca, and Mundipharma. He has received funding for research projects from: GSK and Almirall. He is a chief medical adviser to the charity Asthma UK, a member of the BTS SIGN Asthma guideline group and the NICE Asthma guideline group. DP: board Membership: Aerocrine, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva. Consultancy: Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, and Teva. Grants/Grants Pending: UK National Health Service, British Lung Foundation, Aerocrine, AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline, Meda, Merck, Mundipharma, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva, and Zentiva. Payments for lectures/speaking: Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, SkyePharma, Takeda, and Teva. Payment for manuscript preparation: Mundipharma and Teva. Patents (planned, pending or issued): AKL. Payment for the development of educational materials: GSK and Novartis. Stock/Stock options: Shares in AKL which produces phytopharmaceuticals and owns 80% of Research in Real Life and its subsidiary social enterprise Optimum Patient Care. Payment for travel/accommodations/meeting expenses: Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva. Funding for patient enrolment or completion of research: Almirall, Chiesi, Teva, and Zentiva. Peer reviewer for grant committees: Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012), and HTA (2014). Unrestricted funding for investigator-initiated studies: Aerocrine, AKL, Almirall, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, Teva, and Zentiva. AB is an employee of RiRL, which has conducted paid research in respiratory disease on behalf of the following organisations in the past 5 years: Aerocrine, AKL Ltd, Almirall, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, Teva, and Zentiva. ST, CM, and KR have no conflicts of interest to declare.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Turner, S., Richardson, K., Burden, A. et al. Initial step-up treatment changes in asthmatic children already prescribed inhaled corticosteroids: a historical cohort study. npj Prim Care Resp Med 25, 15041 (2015). https://doi.org/10.1038/npjpcrm.2015.41
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/npjpcrm.2015.41
This article is cited by
-
Applying UK real-world primary care data to predict asthma attacks in 3776 well-characterised children: a retrospective cohort study
npj Primary Care Respiratory Medicine (2018)