Abstract
Age-dependent cognitive decline is a major debilitating event affecting even individuals who are otherwise healthy. Understanding the molecular basis underlying these changes may increase the healthspan of the elderly population. It may also reveal insights into the pathogenesis of numerous neurodegenerative disorders characterized by cognitive deficits, as aging is the major risk factor for most of these disorders. Alzheimer’s disease (AD), the most common neurodegenerative disorder, first manifests itself as deficits in encoding new memories. As AD progresses, these deficits spread to other cognitive domains that further debilitate the person before contributing to their demise. Suppression of the mammalian target of rapamycin (mTOR) increases healthspan and lifespan in several organisms. Numerous reports have linked alterations in mTOR signaling to age-dependent cognitive decline and the pathogenesis of AD. This review will discuss recent work highlighting the complex role of mTOR in cognitive aging and in the pathogenesis of AD.
Similar content being viewed by others
Introduction
People over the age of 65 years represent the fastest growing segment of the population. There will be an estimated two billion people in the world and 88.5 million people in the United States alone, over the age of 60 by the year 2050.1,2 These estimates suggest that aging and age-related diseases should be a top public health concern. Thus, discovering novel treatments that promote healthy aging and decrease the incidence of age-related diseases would have a major and long-lasting beneficial impact on the society.
Overwhelming data suggest that decreasing the activity of the mammalian target of rapamycin (mTOR) increases lifespan and healthspan. This effect has been found in several species, including Saccharomyces cerevisiae, Caenorhabditis elegans, Drosophila melanogaster and rodents.3 There is no direct evidence that inhibiting mTOR activity promotes healthy aging and increases longevity in primates. However, caloric restriction-mediated protection against some age-related changes in primates, including humans, appears to be mediated by reductions in mTOR activity.4,5 These findings suggest that mTOR is a valid molecular target at which to develop new approaches that promote healthy aging.
In addition, mTOR has a role in several neurodegenerative diseases including Alzheimer’s disease (AD), Down syndrome, Huntington’s disease and Parkinson’s disease.6–8 Several laboratories have reported that mTOR is hyperactive in the brains of AD patients, and that this dysregulation may contribute to disease pathogenesis.9–11 However, not all studies agree with this notion.12 This review takes a balanced approach and discusses seminal and current research findings regarding mTOR’s role in brain aging and AD.
Brief overview of mTOR functions
mTOR is a serine/threonine protein kinase, which is involved in the regulation of both protein synthesis and degradation, longevity and cytoskeletal formation.13,14 mTOR is the key catalytic unit of two separate large multimeric protein complexes, mTOR complex 1 (mTORC1) and 2 (mTORC2; Figure 1). mTORC1 consist of mTOR, raptor, the mammalian lethal with sec-18 protein 8 (mLSTR8), the DEP domain–containing mTOR-interacting protein (Deptor), the Tti1/Tel2 complex and the proline-rich Akt substrate 40 kDa (PRAS40).13 mTORC2 consist of mTOR, rictor, mLSTR8, deptor, Tti1/Tel2 and mammalian stress-activated MAP kinase-interacting protein 1.15 mTORC1 is regulated by signaling from insulin, growth factors, amino acids and oxidative stress.16 It promotes cell growth and proliferation (by facilitating mRNA translation and protein synthesis), lipid biogenesis, regulates mitochondrial metabolism and modulates autophagy.17–19 In contrast, mTORC2 appears to be strictly under the control of growth factors and it is thought to function primarily in cytoskeleton assembly and cell size.20,21 Both mTORC1 and mTORC2 are involved in the regulation of longevity.14,16
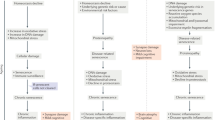
The mTORC1 and mTORC2 have distinct constituent proteins and regulate different downstream targets. Both mTORC1 and mTORC2 share the catalytic mTOR subunit, the mammalian lethal with sec-13 protein 8 (mLST8), the Tti1 and Tel2 complex and the DEP domain–containing mTOR-interacting protein (deptor), all depicted in blue. The mTORC1 contains the rapamycin sensitive raptor subunit (depicted in red) and the proline-rich Akt substrate 40 kDa (PRAS40; depicted in green). Downstream effectors ribosomal protein S6 kinase beta-1 (S6K1) and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) have a critical role in protein translation by regulating the activation of initiation factors. Inhibition of mTORC1 decreases the phosphorylation of ULK1, which initiates the sequential activation of several autophagy-related proteins, culminating in the formation of AVs. In contrast, the mTORC2 contains the rictor subunit (depicted in orange), the protor component (depicted in yellow) and the mammalian stress-activated MAP kinase-interacting protein 1 (sin1; depicted in purple). Downstream effectors of mTORC2 are involved in regulating longevity, cellular stress response and cytoskeletal dynamics. The mTORC1 receives signals from growth factors, glucose, inflammatory cell signaling proteins and extracellular amino acids. The mTORC2 receives signals primarily from growth factors. AV, autophagosome; mTORC, mammalian target of rapamycin complex.
mTORC1 regulates protein translation mainly by controlling the activity of ribosomal protein S6 kinase-1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4EBP1), which directly control the activity of several inhitiation factors.15,16,22,23 Malnutrition, stress, insulin, growth factors and various other signaling pathways that converge on mTOR may differentially activate/inhibit protein synthesis.15,16
Autophagy refers to the process in which old, damaged or misfolded aberrant proteins and/or organelles are degraded and their constituents recycled in lysosomes.17,24,25 Autophagy is important for maintaining proper function of cells within various organs including the brain.22,24,26–28 There are three major subtypes of autophagy: microautophagy, chaperone-mediated autophagy and macroautophagy. While macro- and microautophagy involve the “in bulk” degradation of regions of the cytosol, chaperone-mediated autophagy is a more selective pathway and only proteins with a lysosomal targeting sequence are degraded.25,29,30 In this review, we will focus on macroautophagy as most of the work connecting autophagy to aging and AD is linked to macroautophagy.
Macroautophagy (herein referred to as autophagy) is a multistep process by which proteins and cytosolic organelles are targeted for degradation and sequestered by a double or multi-membrane spherical structure known as an autophagosome (AV). After it is formed, the AV is delivered to the lysosome for degradation.31 mTORC1 is a negative regulator of autophagy.31,32 Indeed, inhibition of mTORC1 decreases the phosphorylation of ULK1, which initiates the sequential activation of several autophagy-related proteins, culminating in the formation of AVs.31–34
mTOR in aging: an overview
The first evidence of TOR’s involvement in aging came from work conducted in S. cerevisiae. Deletion of the gene encoding the yeast orthologue of S6K1 resulted in a doubling of the chronological lifespan.35 Shortly after, inhibition of raptor or S6K1 was shown to extend lifespan in C. elegans.36,37 These initial studies have been confirmed and expanded to other species.14,38 To this end, a landmark report showed that rapamycin, an mTOR inhibitor, fed to genetically heterogeneous mice, increased their lifespan.39 The involvement of mTOR in regulating lifespan in mammals has also been shown using two independent genetic approaches. The first one highlighted that the deletion of S6K1, a downstream target of mTOR, extends lifespan and healthspan in both male and female mice by ~9%.40 The second report found that mice with two hypomorphic alleles that reduce mTOR expression by 25% compared with wild-type levels showed an ~20% increase in the median lifespan.41 Notably, complete inhibition of TOR signaling during development leads to premature lethality,42–44 indicating that TOR signaling is an important and evolutionarily conserved regulator of longevity, which operates within a narrow range in order to maintain homeostasis and health. The role of mTOR in aging has been extensively discussed elsewhere.14,45
mTOR, cognition and brain aging
mTOR is highly expressed throughout the brain, primarily in neurons, but it is also found in astrocytes.46,47 In addition to regulating brain energy levels, mTOR is linked to synaptic plasticity and cognition.18,21,48 For example, mTOR activity is necessary for the expression of the late phase of long-term potentiation in the hippocampus, by modulating de novo protein synthesis after long-term potentiation induction.48,49 In addition, mTOR coordinates the timing and location for the synthesis of new proteins.48,49 A critical role for mTOR in cognition has also been shown by conditionally removing rictor, and therefore mTORC2 activity, from excitatory neurons in limbic and cortical regions after development.50 These conditional rictor knockout mice, which still have a fully functional mTORC1, are incapable of establishing long-term potentiation and consequently show cognitive deficits.50 There deficits appear mediated by alterations in actin-dynamics, which are known to regulate the growth of dendritic spines necessary for memory formation.50
Although mTOR activity is necessary for normal cognition, mTOR hyperactivity is also detrimental to brain function. The primary evidence comes from clinical cases of tuberous sclerosis, in which mTOR hyperactivity leads to cognitive deficits.51 Consistent with these observations, mTOR hyperactivity in mouse models of tuberous sclerosis is linked to synaptic and cognitive deficits.52 Mechanistically, the cognitive deficits in these mice are mediated by an inability to maintain late-phase long-term potentiation, which is regulated by mTOR activity.48,49 Interestingly, reducing mTOR activity via a 2-week administration of rapamycin ameliorates synaptic and cognitive deficits in tuberous sclerosis mice.52 The link between hyperactive mTOR signaling and cognitive dysfunction has been widely confirmed by others.53,54 Currently, there are ongoing clinical trials aimed at determining whether reducing mTOR activity with everolimus and sirolimus (two rapamycin analogs) ameliorates different aspects of tuberous sclerosis symptoms, including brain mTOR hyperactivity and cortical hyperexcitability (ClinicalTrials.gov Identifiers: NCT02451696 and NCT00490789). mTOR is also hyperactive in developmental disorders, such as Down syndrome, Rett syndrome and fragile X syndrome.55
mTOR also has a role in cognitive decline associated with aging. To this end, life-long rapamycin administration ameliorates age-dependent spatial memory deficits in C57Bl/6 mice.56 These rapamycin-mediated improvements were associated with decreased mTOR signaling and brain inflammation, as well as increased hippocampal NMDA signaling.56 Notably, in the same study, mice that were given rapamycin at 15 months of age for 3 months show no detectable changes in cognitive functions.56 These findings have been confirmed by independent studies, which suggest that in addition to cognition, rapamycin also improves anxiety-related behaviors.57,58 Further, the age-dependent decrease in autophagy function may lead to increased protein accumulation, which may interfere with normal brain function.22,26,32,34 Inhibition of mTOR increases autophagy induciton, which presumably maintains cellular function during aging by counteracting the age-dependent protein accumulation. Given the role of mTOR in several signaling pathways, it is plausible that multiple molecular mechanisms might link the reduction of mTOR signaling to improvements in age-dependent cognitive function. Future studies are necessary to establish why reducing mTOR signaling in older mice has no effects on cognition. It is tempting to speculate that there is a critical period of time before, but not after which, changes in brain function that occur during aging can be mitigated by reducing mTOR. Identifying why some age-dependent changes in brain function are reversible and others are not may have long-lasting effects on the field’s understanding of age-dependent cognitive deficits.
In summary, a large body of work spanning decades has revealed that mTOR signaling is an important regulator of protein homeostasis, by simultaneously controlling protein synthesis and protein degradation. Alterations in this delicate balance could disrupt the production of key proteins involved in synaptic plasticity leading to deficits in cognition. In other words, there is clear evidence that either excessive or insufficient mTOR signaling has detrimental effects on cognitive function.
mTOR and AD
Amyloid plaques and neurofibrillary tangles, comprised primarily of amyloid-β (Aβ) and tau, respectively, are the histopathological hallmarks of AD.59 Aβ is generated from the amyloid precursor protein (APP), a soluble transmembrane glycoprotein.59 The β-site APP cleaving enzyme 1 (BACE-1) cleaves APP to generate a C-terminal fragment of 99 amino acids (C99), which is then cleaved by the γ-secretase complex to produce Aβ.59 Presenilin 1 (PS1) or PS2 are the major catalytic unit of the γ-secretase complex.
Tau, in its normal state is a soluble protein, which promotes microtubule assembly and stabilization.60 In addition, tau regulates postsynaptic glutamate receptors, directly interfaces with synaptic signaling proteins and influences the function of synaptic mitochondria.60 Pathological tau, by contrast, exhibits altered solubility properties, is abnormally phosphorylated and forms intracellular inclusions.61–64 Tau accumulation is not unique to AD and is found in other neurodegenerative diseases such as frontotemporal dementia with Parkinsonism, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration.60,65,66 The signaling pathways involved in AD pathogenesis have been reviewed elsewhere.67 In the following sections, we will focus on the interaction between mTOR and the two hallmark lesions of AD, namely Aβ and tau.
Aβ and mTOR
The relationship between Aβ and mTOR has been extensively studied and a complex picture has emerged. Early in vitro reports showed that exposure of mouse neuroblastoma cells to 20 μmol/l Aβ42 for 24 h was sufficient to decrease mTOR signaling.12 However, when applied at lower and more physiological concentrations, Aβ has the opposite effects on mTOR signaling. For example, mTOR is upregulated in chinese hamster ovary (CHO) cells and mouse neuroblastoma cells (N2A) stably transfected with mutant APP.68,69 mTOR hyperactivity was also induced in wild-type N2A cells by application of Aβ25–35.68 In mutant CHO cells, which are known to secrete low concentration of low molecular weight Aβ oligomers,70 the effects on mTOR were prevented by blocking Aβ production.69 Consistent with these findings, intrahippocampal injection of naturally secreted Aβ oligomers was sufficient to increase mTOR signaling in the brains of wild-type mice.71
Work in transgenic mice has also generated conflicting results. To this end, it has been reported that 12-month-old APP/PS1 mice have lower mTOR signaling than age-matched wild-type mice,12 which directly contradicts an earlier report showing hyperactive mTOR signaling in 9-month-old APP/PS1 mice.68 In Tg2576 mice, mTOR signaling is downregulated in young pre-pathological mice. In contrast, in aged Tg2575 mice with established Aβ pathology, mTOR activity is similar to age-matched wild-type mice.72 Other reports have shown an age- and regional-dependent increase in mTOR signaling in 3xTg-AD mice.69,71 Notably, genetically or immunologically preventing Aβ accumulation was sufficient to reduce mTOR signaling to wild-type levels, indicating that mTOR hyperactivity was due to Aβ accumulation.71 The results in the 3xTg-AD mice are in agreement with studies in postmortem human AD brains, which consistently show an upregulation of mTOR signaling.73–78
Although the mechanisms by which Aβ alters mTOR activity remain elusive, confocal microscopy data showed a direct interaction between intraneuronal Aβ42 and mTOR.72 Furthermore, the Aβ-mediated increase in mTOR activity can be prevented by blocking the phosphorylation of PRAS40, suggesting that the build-up of Aβ may facilitate PRAS40 phosphorylation.71 Consistent with this observation, the steady-state levels of phosphorylated PRAS40 were significantly higher in the brains of 3xTg-AD mice.71 In summary, a large body of evidence suggests a direct or indirect interaction between Aβ and mTOR; however, this picture is complex as both in vivo and in vitro work have often revealed opposite effects. Although it is hard to dissect the causes explaining these divergent effects, strain and age of the mice, as well as different levels of Aβ can have differential effects on mTOR. For example, mTOR hyperactivity in 3xTg-AD mice precedes the formation of Aβ plaques and it is most likely due to high soluble Aβ levels.69 In contrast, in APP/PS1 mice mTOR hyperactivity has been reported when the mice have widespread Aβ plaque deposits throughout the brain.12
Recent evidence suggests that, just as Aβ affects mTOR, mTOR also affects Aβ.10 This indicates that the two proteins interact closely with one another and elucidating the mechanism(s) of this interaction may reveal previously unknown aspects of AD pathogenesis. The first in vivo evidence indicating that modulation of mTOR signaling had a direct effect on Aβ pathology came from pharmacological studies using 3xTg-AD mice. Specifically, 3xTg-AD and wild-type mice were given rapamcyin starting at the onset of cognitive deficits, for 10 weeks.69 Rapamycin restored the hyperactive mTOR signaling in 3xTg-AD mice to control levels, rescued cognitive deficits and decreased Aβ and tau pathology.69 This study highlighted a crosstalk between Aβ and mTOR as it demonstrated that reducing high levels of mTOR activity reduced Aβ deposition, just as the application of Aβ increased mTOR activity. Consistent with this finding, reducing mTOR signaling by rapamycin or temsirolimus, ameliorated AD-like pathology and cognitive deficits in hAPP(J20) mice or in APP/PS1 mice, respectively.79,80 Furthermore, rapamycin also reduced the formation of Aβ plaques and tangles when administered prior to their formation.81 Conversely, administration of rapamycin to 15-month-old 3xTg-AD mice, with established AD-like neuropathology, had no effect on cognitive deficits or plaque and tangle load.81 The rapamycin-mediated reduction in AD neuropathology was linked to an increase in autophagy induction,69,81 which may explain why rapamycin administration to mice with established pathology does not decrease Aβ or tau pathology. To this end, elegant work by the Nixon laboratory has shown that AVs accumulate in human AD brains as well as in a mouse model of AD, suggesting a deficit in their clearance.82,83 Consistent with this theory, inducing autophagy after the deficit in autophagy flux occurs (most likely following AD-like neuropahtology) would simply increase AV formation, which would fail to fuse to lysosomes for content degradation.10,25 Indeed, compelling evidence suggests that substrate-filled AVs drastically accumulate in AD and animal models.81–83 Thus, increasing autophagy induciton (e.g., by rapamycin) may further lead to the accumulation of AVs, which may exacerbate AD pathogenesis as Aβ can be generated in and released from these vesicles.25
Genetic studies strengthened the link between mTOR and AD pathogenesis. To this end, genetically and selectively reducing mTOR signaling in the brains of Tg2576 mice was sufficient to rescue memory deficits. This rescue of cognitive deficits was associated with reduced Aβ deposits and a change in the abnormal pattern of hippocampal gene expression of the Tg2576 mice to a more similar pattern found in wild-type control mice.84 Collectively, these studies suggest that hyperactive mTOR in AD contributes to the accumulation of Aβ.
Tau and mTOR
The evidence linking mTOR to tau is less controversial and several laboratories have consistently shown that hyperactive mTOR contributes to tau pathology. In postmortem human AD brains, hyperactive mTOR signaling was found in neurons that were predicted to develop tau pathology.73 Work in animal models has confirmed and expanded on this initial observation. Hyperactive TOR in Drosophila facilitates the development of tau pathology and the associated neurodegeneration.85 Consistent with these observations, blocking TOR signaling rescued tau-induced toxicity, while genetically increasing TOR signaling enhanced tau-induced toxicity in Drosophila.86 Mice with hyperactive mTOR also have increased brain levels of total and phosphorylated tau.87 Conversely, reducing mTOR has beneficial effects on tau pathology. To this end, reducing mTOR with rapamcyin in a transgenic mouse expressing mutant human tau decreased tau pathology and improved the associated motor deficits.87 Similar to these observations, chronic treatment with the rapamycin ester CCI-779/Temsirolimus in Tg30 mutant tau mice, decreased mTOR signaling, stimulated autophagy, reduced tau levels and neurofibrillary tangle density, which led to an attenution of motor deficits.88
The mechanism underlying these observations is likely multifactorial. For example, hyperactive mTOR signaling decreased autophagy turnover, which is a known degradation pathway for tau.89,90 mTOR can also regulate tau levels by increasing translation of its mRNA. Indeed, direct evidence from primary hippocampal neurons showed that inhibition of mTOR by rapamycin suppresses tau translation, while constitutively active mTOR signaling increased tau translation.91 In addition, mTOR can directly regulate tau phosphorylation. To this end, mTOR and S6K1 phosphorylate tau at multiple residues.92–94 mTOR also suppresses the activity of the protein phosphatase 2A, an enzyme known to remove phosphate groups from tau.13,95 One startling implication of these observations is that long-term exposure to hyperactive mTOR might increase tau translation and decrease its degradation/turnover, while concomitantly increasing tau phosphorylation. Collectively, these studies highlight multiple pathways by which mTOR signaling contributes to tau pathology.
Concluding remarks
We propose a crosstalk between mTOR and the two neuropathological hallmarks of AD, Aβ and tau (Figure 2). Specifically, Aβ accumulation increases mTOR signaling, which in turn further increases Aβ accumulation by decreasing autophagy induction. mTOR regulates tau pathology by controlling its phosphorylation and degradation (via autophagy). Furthermore, mTOR directly increases overall tau levels by regulating translation of its mRNA. This model predicts that factors (e.g., environment, genetic) known to increase mTOR may contribute to the development of AD. Consistent with this prediction, several AD risk factors, such as high fat diet, diabetes, traumatic brain injury and the inheritance of the apolipoprotein E4 gene, also increase mTOR activity. In summary, mTOR plays a key role in aging and AD pathogenesis. Therefore, modulating mTOR activity is an attractive avenue to discover new therapies to enhance healthy brain and body aging, as well as attenuating age-related disease progression and treating age-related diseases, including AD.
Diagram illustrating the proposed crosstalk among mTOR, Aβ and tau. In AD, hyperactive mTOR increases Aβ and tau production, Aβ positively feedbacks on to mTOR further increasing mTOR activity. Many factors including diabetes, traumatic brain injury and ApoE4 may all influence the crosstalk of these proteins and the aberrant cycle they create leading to AD pathogenesis. AD, Alzheimer’s disease; Aβ, amyloid-β; ApoE4, apolipoprotein E4; mTOR, mammalian target of rapamycin.
References
Vincent GK, Velkoff VA, U.S. Census Bureau. The Next Four Decades: the Older Population in the United States: 2010 to 2050. U.S. Department of Commerce, Economics and Statistics Administration: U.S. Census Bureau, Washington, DC, USA, 2010.
Harper S . Economic and social implications of aging societies. Science 2014; 346: 587–591.
Lamming DW, Ye L, Sabatini DM, Baur JA . Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest 2013; 123: 980–989.
Blagosklonny MV . Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans). Cell Cycle 2010; 9: 683–688.
Holloszy JO, Fontana L . Caloric restriction in humans. Exp Gerontol 2007; 42: 709–712.
Lee JH, Tecedor L, Chen YH, Monteys AM, Sowada MJ, Thompson LM et al. Reinstating aberrant mTORC1 activity in Huntington's disease mice improves disease phenotypes. Neuron 2015; 85: 303–315.
Bove J, Martinez-Vicente M, Vila M . Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci 2011; 12: 437–452.
Iyer AM, van Scheppingen J, Milenkovic I, Anink JJ, Adle-Biassette H, Kovacs GG et al. mTOR Hyperactivation in down syndrome hippocampus appears early during development. J Neuropathol Exp Neurol 2014; 73: 671–683.
Cai Z, Zhao B, Li K, Zhang L, Li C, Quazi SH et al. Mammalian target of rapamycin: a valid therapeutic target through the autophagy pathway for Alzheimer's disease? J Neurosci Res 2012; 90: 1105–1118.
Oddo S . The role of mTOR signaling in Alzheimer disease. Front Biosci 2012; 4: 941–952.
Wang C, Yu JT, Miao D, Wu ZC, Tan MS, Tan L . Targeting the mTOR signaling network for Alzheimer's disease therapy. Mol Neurobiol 2014; 49: 120–135.
Lafay-Chebassier C, Paccalin M, Page G, Barc-Pain S, Perault-Pochat MC, Gil R et al. mTOR/p70S6k signalling alteration by Abeta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer's disease. J Neurochem 2005; 94: 215–225.
Wullschleger S, Loewith R, Hall MN . TOR signaling in growth and metabolism. Cell 2006; 124: 471–484.
Johnson SC, Rabinovitch PS, Kaeberlein M . mTOR is a key modulator of ageing and age-related disease. Nature 2013; 493: 338–345.
Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev 1999; 13: 1422–1437.
Hay N, Sonenberg N . Upstream and downstream of mTOR. Genes Dev 2004; 18: 1926–1945.
Kim YC, Guan KL . mTOR: a pharmacologic target for autophagy regulation. J Clin Invest 2015; 125: 25–32.
Perluigi M, Di Domenico F, Butterfield DA . mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis 2015. e-pub ahead of print19 March 2015. doi: 10.1016/j.nbd.2015.03.014.
Wang X, Proud CG . The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006; 21: 362–369.
Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol 2006; 16: 1865–1870.
Graber TE, McCamphill PK, Sossin WS . A recollection of mTOR signaling in learning and memory. Learn Mem 2013; 20: 518–530.
Hands SL, Proud CG, Wyttenbach A . mTOR's role in ageing: protein synthesis or autophagy? Aging (Albany NY) 2009; 1: 586–597.
Ruvinsky I, Meyuhas O . Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 2006; 31: 342–348.
Klionsky DJ . The autophagy connection. Dev Cell 2010; 19: 11–12.
Nixon RA, Yang DS . Autophagy failure in Alzheimer's disease--locating the primary defect. Neurobiol Dis 2011; 43: 38–45.
Martinez-Lopez N, Athonvarangkul D, Singh R . Autophagy and aging. Adv Exp Med Biol 2015; 847: 73–87.
Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 2010; 90: 1383–1435.
Zhang C, Cuervo AM . Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med 2008; 14: 959–965.
Mizushima N, Tsukamoto S, Kuma A . [Autophagy in embryogenesis and cell differentiation]. Tanpakushitsu Kakusan Koso 2008; 53: 2170–2174.
Nah J, Yuan J, Jung YK . Autophagy in neurodegenerative diseases: from mechanism to therapeutic approach. Mol Cells 2015; 38: 381–389.
He C, Klionsky DJ . Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 2009; 43: 67–93.
Jung CH, Ro SH, Cao J, Otto NM, Kim DH . mTOR regulation of autophagy. FEBS Lett 2010; 584: 1287–1295.
Noda NN, Inagaki F . Mechanisms of Autophagy. Annu Rev Biophys 2015; 44: 101–122.
Rubinsztein DC, Marino G, Kroemer G . Autophagy and aging. Cell 2011; 146: 682–695.
Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD . Regulation of longevity and stress resistance by Sch9 in yeast. Science 2001; 292: 288–290.
Jia K, Chen D, Riddle DL . The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 2004; 131: 3897–3906.
Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F . Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 2003; 426: 620.
Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S . Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 2004; 14: 885–890.
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009; 460: 392–395.
Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 2009; 326: 140–144.
Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep 2013; 4: 913–920.
Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G . Drosophila S6 kinase: a regulator of cell size. Science 1999; 285: 2126–2129.
Oldham S, Montagne J, Radimerski T, Thomas G, Hafen E . Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev 2000; 14: 2689–2694.
Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F et al. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol 2004; 24: 6710–6718.
Richardson A, Galvan V, Lin AL, Oddo S . How longevity research can lead to therapies for Alzheimer's disease: The rapamycin story. Exp Gerontol 2014; 68: 51–58.
Li CY, Li X, Liu SF, Qu WS, Wang W, Tian DS . Inhibition of mTOR pathway restrains astrocyte proliferation, migration and production of inflammatory mediators after oxygen-glucose deprivation and reoxygenation. Neurochem Int 2015; 83-84: 9–18.
Meijer AJ, Lorin S, Blommaart EF, Codogno P . Regulation of autophagy by amino acids and MTOR-dependent signal transduction. Amino Acids 2014; 47: 2037–2063.
Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM . A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA 2002; 99: 467–472.
Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W et al. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci USA 2003; 100: 14368–14373.
Huang W, Zhu PJ, Zhang S, Zhou H, Stoica L, Galiano M et al. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci 2013; 16: 441–448.
Ehninger D, de Vries PJ, Silva AJ . From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. J Intellect Disabil Res 2009; 53: 838–851.
Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med 2008; 14: 843–848.
Costa-Mattioli M, Monteggia LM . mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat Neurosci 2013; 16: 1537–1543.
Ricciardi S, Boggio EM, Grosso S, Lonetti G, Forlani G, Stefanelli G et al. Reduced AKT/mTOR signaling and protein synthesis dysregulation in a Rett syndrome animal model. Hum Mol Genet 2011; 20: 1182–1196.
Troca-Marin JA, Alves-Sampaio A, Montesinos ML . Deregulated mTOR-mediated translation in intellectual disability. Prog Neurobiol 2012; 96: 268–282.
Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell 2012; 11: 326–335.
Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience 2012; 223: 102–113.
Kolosova NG, Vitovtov AO, Muraleva NA, Akulov AE, Stefanova NA, Blagosklonny MV . Rapamycin suppresses brain aging in senescence-accelerated OXYS rats. Aging (Albany NY) 2013; 5: 474–484.
Querfurth HW, LaFerla FM . Alzheimer's disease. N Engl J Med 2010; 362: 329–344.
Ballatore C, Lee VM, Trojanowski JQ . Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci 2007; 8: 663–672.
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI . Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 1986; 83: 4913–4917.
Ihara Y, Nukina N, Miura R, Ogawara M . Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer's disease. J Biochem 1986; 99: 1807–1810.
Kosik KS, Joachim CL, Selkoe DJ . Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA 1986; 83: 4044–4048.
Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A . Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci USA 1988; 85: 4051–4055.
Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998; 393: 702–705.
Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B . Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 1998; 95: 7737–7741.
Godoy JA, Rios JA, Zolezzi JM, Braidy N, Inestrosa NC . Signaling pathway cross talk in Alzheimer's disease. Cell Commun Signal 2014; 12: 23.
Zhou XW, Tanila H, Pei JJ . Parallel increase in p70 kinase activation and tau phosphorylation (S262) with Abeta overproduction. FEBS Lett 2008; 582: 159–164.
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S . Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem 2010; 285: 13107–13120.
Koo EH, Squazzo SL . Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem 1994; 269: 17386–17389.
Caccamo A, Maldonado MA, Majumder S, Medina DX, Holbein W, Magri A et al. Naturally secreted amyloid-beta increases mammalian target of rapamycin (mTOR) activity via a PRAS40-mediated mechanism. J Biol Chem 2011; 286: 8924–8932.
Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, Lin MT et al. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PLoS ONE 2010; 5.
An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K et al. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer's disease. Am J Pathol 2003; 163: 591–607.
Chang RC, Wong AK, Ng HK, Hugon J . Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer's disease. Neuroreport 2002; 13: 2429–2432.
Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer's disease pathology. J Neurochem 2005; 93: 105–117.
Onuki R, Bando Y, Suyama E, Katayama T, Kawasaki H, Baba T et al. An RNA-dependent protein kinase is involved in tunicamycin-induced apoptosis and Alzheimer's disease. EMBO J 2004; 23: 959–968.
Pei JJ, Bjorkdahl C, Zhang H, Zhou X, Winblad B . p70 S6 kinase and tau in Alzheimer's disease. J Alzheimers Dis 2008; 14: 385–392.
Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R et al. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem 2015; 133: 739–749.
Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS ONE 2010; 5: e9979.
Jiang T, Yu JT, Zhu XC, Tan MS, Wang HF, Cao L et al. Temsirolimus promotes autophagic clearance of amyloid-beta and provides protective effects in cellular and animal models of Alzheimer's disease. Pharmacol Res 2014; 81: 54–63.
Majumder S, Richardson A, Strong R, Oddo S . Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS ONE 2011; 6: e25416.
Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH et al. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol 2005; 171: 87–98.
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci 2008; 28: 6926–6937.
Caccamo A, De Pinto V, Messina A, Branca C, Oddo S . Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer's disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J Neurosci 2014; 34: 7988–7998.
Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB . TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol 2006; 16: 230–241.
Steinhilb ML, Dias-Santagata D, Fulga TA, Felch DL, Feany MB . Tau phosphorylation sites work in concert to promote neurotoxicity in vivo . Mol Biol Cell 2007; 18: 5060–5068.
Caccamo A, Magri A, Medina DX, Wisely EV, Lopez-Aranda MF, Silva AJ et al. mTOR regulates tau phosphorylation and degradation: implications for Alzheimer's disease and other tauopathies. Aging Cell 2013; 12: 370–380.
Frederick C, Ando K, Leroy K, Heraud C, Suain V, Buee L et al. Rapamycin Ester Analog CCI-779/Temsirolimus Alleviates Tau Pathology and Improves Motor Deficit in Mutant Tau Transgenic Mice. J Alzheimers Dis 2014; 44: 1145–1156.
Lee MJ, Lee JH, Rubinsztein DC . Tau degradation: the ubiquitin-proteasome system versus the autophagy-lysosome system. Prog Neurobiol 2013; 105: 49–59.
Wang Y, Mandelkow E . Degradation of tau protein by autophagy and proteasomal pathways. Biochem Soc Trans 2012; 40: 644–652.
Morita T, Sobue K . Specification of neuronal polarity regulated by local translation of CRMP2 and Tau via the mTOR-p70S6K pathway. J Biol Chem 2009; 284: 27734–27745.
Pei JJ, An WL, Zhou XW, Nishimura T, Norberg J, Benedikz E et al. P70 S6 kinase mediates tau phosphorylation and synthesis. FEBS Lett 2006; 580: 107–114.
Tang Z, Bereczki E, Zhang H, Wang S, Li C, Ji X et al. Mammalian target of rapamycin (mTor) mediates tau protein dyshomeostasis: implication for Alzheimer disease. J Biol Chem 2013; 288: 15556–15570.
Tang Z, Ioja E, Bereczki E, Hultenby K, Li C, Guan Z et al. mTor mediates tau localization and secretion: Implication for Alzheimer's disease. Biochim Biophys Acta 2015; 1853: 1646–1657.
Liu C, Gotz J . How it all started: tau and protein phosphatase 2A. J Alzheimers Dis 2013; 37: 483–494.
Acknowledgements
This work was supported by grants to S.O. by the National Institutes of Health (R01 AG037637).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Talboom, J., Velazquez, R. & Oddo, S. The mammalian target of rapamycin at the crossroad between cognitive aging and Alzheimer’s disease. npj Aging Mech Dis 1, 15008 (2015). https://doi.org/10.1038/npjamd.2015.8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/npjamd.2015.8
This article is cited by
-
Impact of Sleep Deprivation on Major Neuroinflammatory Signal Transduction Pathways
Sleep and Vigilance (2022)
-
Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration
Molecular Neurodegeneration (2021)
-
A transition to degeneration triggered by oxidative stress in degenerative disorders
Molecular Psychiatry (2021)
-
The Dephosphorylation of p70S6 (Thr389) Kinase as a Marker of l-Glutamate-Induced Excitotoxicity Related to Diabetes Disturbances—an Unconventional In Vitro Model
Neurotoxicity Research (2020)
-
iPSC-derived familial Alzheimer’s PSEN2 N141I cholinergic neurons exhibit mutation-dependent molecular pathology corrected by insulin signaling
Molecular Neurodegeneration (2018)