Abstract
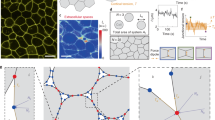
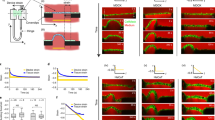
Mechanical interactions play a crucial role in epithelial morphogenesis, yet understanding the complex mechanisms through which stress and deformation affect cell behaviour remains an open problem. Here we formulate and analyse the active tension network (ATN) model, which assumes that the mechanical balance of cells within a tissue is dominated by cortical tension and introduces tension-dependent active remodelling of the cortex. We find that ATNs exhibit unusual mechanical properties. Specifically, an ATN behaves as a fluid at short times, but at long times supports external tension like a solid. Furthermore, an ATN has an extensively degenerate equilibrium mechanical state associated with a discrete conformal—‘isogonal’—deformation of cells. The ATN model predicts a constraint on equilibrium cell geometries, which we demonstrate to approximately hold in certain epithelial tissues. We further show that isogonal modes are observed in the fruit fly embryo, accounting for the striking variability of apical areas of ventral cells and helping understand the early phase of gastrulation. Living matter realizes new and exotic mechanical states, the study of which helps to understand biological phenomena.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bellaiche, Y. & Heisenberg, C. Forces in tissue morphogenesis and patterning. Cell 153, 948–962 (2013).
Farhadifar, R., Roper, J. C., Aigouy, B., Eaton, S. & Julicher, F. The influence of cell mechanics, cell–cell interactions, and the proliferation of epithelial packing. Curr. Biol. 17, 2095–2104 (2007).
Rauzi, M., Verant, P., Lecuit, T. & Lenne, P. F. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol. 10, 1401–1410 (2008).
He, B., Doubrovinski, K., Polyakov, O. & Wieschaus, E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 508, 392–396 (2014).
Lecuit, T. & Lenne, P. F. Cell surface mechanics and the control of cell shape, tissue patterns, and morphogenesis. Nat. Rev. Mol. Cell Biol. 8, 633–644 (2007).
Nelson, C. et al. Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl Acad. Sci. USA 102, 11594–11599 (2005).
Shraiman, B. Mechanical feedback as a possible regulator of tissue growth. Proc. Natl Acad. Sci. USA 102, 3318–3323 (2005).
Fernandez-Gonzalez, R., Simeos, M., Roper, J. C., Eaton, S. & Zallen, J. Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736–743 (2009).
MacKintosh, F. C. & Levine, A. J. Nonequilibrium mechanics and dynamics of motor-activated gels. Phys. Rev. Lett. 100, 018104 (2008).
Wang, N. et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 282, 606–616 (2002).
Salbreux, G., Charras, G. & Paluch, E. Actin cortex mechanics and cellular morphogenesis. Cell 22, 536–545 (2012).
Hartsock, A. & Nelson, W. J. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778, 660–669 (2008).
Wozniak, M. & Chen, C. Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell Biol. 10, 34–43 (2009).
Kasza, K. et al. The cell as a material. Curr. Opin. Cell Biol. 19, 101–107 (2007).
Honda, H. Geometric models for cells in tissues. Int. Rev. Cytol. 81, 191–248 (1983).
Chiou, K., Hufnagel, L. & Shraiman, B. Mechanical stress inference for two dimensional cell arrays. PLoS Comp. Biol. 8, e1002512 (2012).
Marchetti, M. C. et al. Hydrodynamics of soft active matter. Rev. Mod. Phys. 85, 1144–1189 (2013).
Maxwell, J. C. On the calculation of the equilibrium and stiffness of frames. Philos. Mag. 27, 294–299 (1864).
Henkes, S., OHern, C. S. & Chakraborty, B. Entropy and temperature of a static granular assembly: an ab initio approach. Phys. Rev. Lett. 99, 038002 (2007).
Choi, W. et al. Remodeling the zonula adherens in response to tension and the role of afadin in this reponse. J. Cell Biol 213, 243–260 (2016).
Cavey, M. & Lecuit, T. Molecular bases of cell–cell junctions stability and dynamics. Cold Spring Harb. Perspect. Biol. 1, a002998 (2009).
Clemen, A. et al. Force-dependent stepping kinetics of myosin-V. Biophys. J. 88, 4402–4410 (2005).
Norstrom, M., Smithback, P. A. & Rock, R. Unconventional processive mechanics of non-muscle myosin IIB. J. Biol. Chem. 285, 26326–26334 (2010).
Kolomeisky, A. B. & Fisher, M. Molecular motors: a theorist’s perspective. Annu. Rev. Phys. Chem. 58, 675–695 (2007).
Pouille, P. A., Ahmadi, P., Brunet, A. C. & Farge, E. Mechanical signals trigger myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal 2, ra16 (2009).
Villain, J. in Ordering in Strongly Fluctuating Condensed Matter Systems (ed. Riste, T.) 221 (Plenum, 1980).
Sweeton, D., Parks, S., Costa, M. & Wieschaus, E. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development 112, 775–789 (1991).
Martin, A. C., Kaschube, M. & Wieschaus, E. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499 (2009).
Massey, F. Jr The Kolmogorov–Smirnov test for goodness of fit. J. Am. Stat. Assoc. 46, 68–78 (1951).
Rauskolb, C. et al. Cytoskeletal tension inhibits hippo signaling through an Ajuba-Warts complex. Cell 158, 143–156 (2014).
Morin, X., Daneman, R., Zavortink, M. & Chia, W. A protein trap strategy to detect GFP-tagged proteins expressed in their endogenous loci in Drosophila. Proc. Natl Acad. Sci. USA 98, 15050–15055 (2002).
Martin, A. C., Gelbart, M., Fernandez-Gonzalez, R., Kaschube, M. & Wieschaus, E. Integration of contractile forces during tissue invagination. J. Cell Biol. 188, 735–749 (2010).
Acknowledgements
The authors gratefully acknowledge stimulating discussions with K. Irvine, T. Lecuit and E. Wieschaus, and thank K. Irvine for sharing the wing imaginal disc data. This work was supported by the NSF PHY-1220616 (B.I.S., N.N.) and PHY-1125915 (M.M.), GBMF grant #2919 (B.I.S./I.H.) and NICHD 5K99HD088708-02 (S.J.S.).
Author information
Authors and Affiliations
Contributions
Model formulation and analysis: B.I.S., I.H., M.M. and N.N. Experimental data: S.J.S. Numerical simulations and data analysis: N.N. Manuscript: B.I.S. and N.N. All authors discussed the results and implications of the work as well as provided critical comments on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1012 kb)
Rights and permissions
About this article
Cite this article
Noll, N., Mani, M., Heemskerk, I. et al. Active tension network model suggests an exotic mechanical state realized in epithelial tissues. Nature Phys 13, 1221–1226 (2017). https://doi.org/10.1038/nphys4219
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys4219
This article is cited by
-
Minimally rigid clusters in dense suspension flow
Nature Physics (2024)
-
Discrete network models of endothelial cells and their interactions with the substrate
Biomechanics and Modeling in Mechanobiology (2024)
-
Patterned mechanical feedback establishes a global myosin gradient
Nature Communications (2022)
-
Modeling epithelial tissues as active-elastic sheets reproduce contraction pulses and predict rip resistance
Communications Physics (2021)
-
Embryonic tissues as active foams
Nature Physics (2021)