Abstract
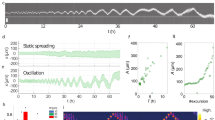
The dispersal of cells from an initially constrained location is a crucial aspect of many physiological phenomena, ranging from morphogenesis to tumour spreading. In such processes, cell–cell interactions may deeply alter the motion of single cells, and in turn the collective dynamics. While contact phenomena like contact inhibition of locomotion are known to come into play at high densities, here we focus on the little explored case of non-cohesive cells at moderate densities. We fully characterize the spreading of micropatterned colonies of Dictyostelium discoideum cells from the complete set of individual trajectories. From data analysis and simulation of an elementary model, we demonstrate that contact interactions act to speed up the early population spreading by promoting individual cells to a state of higher persistence, which constitutes an as-yet unreported contact enhancement of locomotion. Our findings also suggest that the current modelling paradigm of memoryless active particles may need to be extended to account for the history-dependent internal state of motile cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Friedl, P. & Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445–457 (2009).
Friedl, P. & Wolf, K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer 3, 362–374 (2003).
Carmona-Fontaine, C. et al. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 456, 957–961 (2008).
Selmeczi, D. et al. Cell motility as random motion: a review. Eur. Phys. J. Spec. Top. 157, 1–15 (2008).
Li, L., Norrelkke, S. F. & Cox, E. C. Persistent cell motion in the absence of external signals: a search strategy for eukaryotic cells. PLoS ONE 3, e2093 (2008).
Kolmogorov, A., Petrovskii, I. & Piscounov, N. A study of the diffusion equation with increase in the amount of substance, and its application to a biological problem. Math. Mech. 1, 1–25 (1937).
Simpson, M. J. et al. Quantifying the roles of cell motility and cell proliferation in a circular barrier assay. J. R. Soc. Interface 10, 20130007 (2013).
Sengers, B. G., Please, C. P. & Oreffo, R. O. C. Experimental characterization and computational modelling of two-dimensional cell spreading for skeletal regeneration. J. R. Soc. Interface 4, 1107–1117 (2007).
Marel, A. K. et al. Flow and diffusion in channel-guided cell migration. Biophys. J. 107, 1054–1064 (2014).
Golé, L., Rivière, C., Hayakawa, Y. & Rieu, J. P. A quorum-sensing factor in vegetative Dictyostelium discoideum cells revealed by quantitative migration analysis. PLoS ONE 6, 1–9 (2011).
Phillips, J. & Gomer, R. A secreted protein is an endogenous chemorepellant in Dictyostelium discoideum. Proc. Natl Acad. Sci. USA 109, 10990–10995 (2012).
Angelini, T. E., Hannezo, E., Trepat, X., Fredberg, J. J. & Weitz, D. A. Cell migration driven by cooperative substrate deformation patterns. Phys. Rev. Lett. 104, 168104 (2010).
Abercrombie, M. & Heaysman, J. E. Observations on the social behaviour of cells in tissue culture: I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp. Cell Res. 5, 111–131 (1953).
Stramer, B. A. & Mayor, R. Mechanisms and in vivo functions of contact inhibition of locomotion. Nat. Rev. Mol. Cell Biol. 118, 43–55 (2016).
Dyson, L. & Baker, R. E. The importance of volume exclusion in modelling cellular migration. J. Math. Biol. 71, 679–711 (2014).
Serra-Picamal, X. et al. Mechanical waves during tissue expansion. Nat. Phys. 8, 628–634 (2012).
Nnetu, K. D., Knorr, M., Strehe, D., Zink, M. & Käs, J. A. Directed persistent motion maintains sheet integrity during multi-cellular spreading and migration. Soft Matter 8, 6913–6921 (2012).
Yates, C. A., Parker, A. & Baker, R. E. Incorporating pushing in exclusion-process models of cell migration. Phys. Rev. E 91, 052711 (2015).
Sepúlveda, N. et al. Collective cell motion in an epithelial sheet can be quantitatively described by a stochastic interacting particle model. PLoS Comput. Biol. 9, e1002944 (2013).
Petitjean, L. et al. Velocity fields in a collectively migrating epithelium. Biophys. J. 98, 1790–1800 (2010).
Tambe, D. T. et al. Collective cell guidance by cooperative intercellular forces. Nat. Mat. 10, 469–475 (2011).
Coburn, L., Cerone, L., Torney, C., Couzin, I. D. & Neufeld, Z. Interactions lead to coherent motion and enhanced chemotaxis of migrating Cells. Phys. Biol. 10, 046002 (2013).
Duclos, G., Garcia, S., Yevick, H. G. & Silberzan, P. Perfect nematic order in confined monolayers of spindle-shaped cells. Soft Matter 10, 2346–2353 (2014).
Londono, C. et al. Nonautonomous contact guidance signaling during collective cell migration. Proc. Natl Acad. Sci. USA 111, 1807–1812 (2014).
Angelini, T. E. et al. Glass-like dynamics of collective cell migration. Proc. Natl Acad. Sci. USA 108, 4714–4719 (2011).
Park, J.-A. et al. Unjamming and cell shape in the asthmatic airway epithelium. Nat. Mat. 14, 1040–1049 (2015).
Garcia, S. et al. Physics of active jamming during collective cellular motion in a monolayer. Proc. Natl Acad. Sci. USA 112, 15314–15319 (2015).
Vedel, S., Tay, S., Johnston, D. M., Bruus, H. & Quake, S. R. Migration of cells in a social context. Proc. Natl Acad. Sci. USA 110, 129–134 (2013).
Fily, Y. & Marchetti, M. C. Athermal phase separation of self-propelled particles with no alignment. Phys. Rev. Lett. 108, 235702 (2012).
Friedl, P. & Wolf, K. Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 188, 11–19 (2010).
Artemenko, Y., Lampert, T. J. & Devreotes, P. N. Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes. Cell. Mol. Life Sci. 71, 3711–3747 (2014).
Friedl, P., Borgmann, S. & Bröcker, E. B. Amoeboid leukocyte crawling through extracellular matrix: lessons from the Dictyostelium paradigm of cell movement. J. Leukoc. Biol. 70, 491–509 (2001).
Levine, H. Learning physics of living systems from Dictyostelium. Phys. Biol. 11, 053011 (2014).
Coates, J. C. & Harwood, A. J. Cell–cell adhesion and signal transduction during Dictyostelium development. J. Cell Sci. 114, 4349–4358 (2001).
Poujade, M. et al. Collective migration of an epithelial monolayer in response to a model wound. Proc. Natl Acad. Sci. USA 104, 15988–15993 (2007).
Cates, M. E. & Tailleur, J. When are active Brownian particles and run-and-tumble particles equivalent? consequences for motility-induced phase separation. Europhys. Lett. 101, 20010 (2013).
Bosgraaf, L. & Van Haastert, P. J. M. The ordered extension of pseudopodia by amoeboid cells in the absence of external cues. PLoS ONE 4, e5253 (2009).
Peruani, F., Deutsch, A. & Bär, A. Nonequilibrium clustering of self-propelled rods. Phys. Rev. E 74, 030904 (2006).
Peruani, F. et al. Collective motion and nonequilibrium cluster formation in colonies of gliding bacteria. Phys. Rev. Lett. 108, 098102 (2012).
Solon, A. P. et al. Pressure and phase equilibria in interacting active Brownian spheres. Phys. Rev. Lett. 114, 198301 (2015).
Bruna, M. & Chapman, S. J. Excluded-volume effects in the diffusion of hard spheres. Phys. Rev. E 85, 011103 (2012).
Kaiser, D. Bacterial swarming: a re-examination of cell-movement patterns. Curr. Biol. 17, R561–R570 (2007).
Kaiser, D. & Crosby, C. Cell movement and its coordination in swarms of Myxococcus xanthus. Cell Motil. 3, 227–245 (1983).
Patra, P., Kissoon, K., Cornejo, I., Kaplan, H. B. & Igoshin, O. A. Colony expansion of socially motile Myxococcus xanthus cells is driven by growth, motility, and exopolysaccharide production. PLoS Comput. Biol. 12, e1005010 (2016).
Potdar, A. A., Jeon, J., Weaver, A. M., Quaranta, V. & Cummings, P. T. Human mammary epithelial cells exhibit a bimodal correlated random walk pattern. PLoS ONE 5, e9636 (2010).
Li, L., Cox, E. C. & Flyvbjerg, H. ‘Dicty dynamics’: Dictyostelium motility as persistent random motion. Phys. Biol. 8, 046006 (2011).
Bénichou, O., Loverdo, C., Moreau, M. & Voituriez, R. Intermittent search strategies. Rev. Mod. Phys. 83, 81–129 (2011).
Metzner, C. et al. Superstatistical analysis and modelling of heterogeneous random walks. Nat. Commun. 6, 7516 (2015).
Lavi, I., Piel, M., Lennon-Duménil, A.-M., Voituriez, R. & Gov, N. S. Deterministic patterns in cell motility. Nat. Phys. 12, 1146–1152 (2016).
Zimmermann, J., Camley, B. A., Rappel, W.-J. & Levine, H. Contact inhibition of locomotion determines cell–cell and cell-substrate forces in tissues. Proc. Natl Acad. Sci. USA 113, 2660–2665 (2016).
Ramdya, P. et al. Mechanosensory interactions drive collective behaviour in Drosophila. Nature 519, 233–236 (2015).
Roycroft, A. & Mayor, R. Molecular basis of contact inhibition of locomotion. Cell. Mol. Life Sci. 73, 1119–1130 (2016).
Etzrodt, M. et al. Time-resolved responses to chemoattractant, characteristic of the front and tail of Dictyostelium cells. FEBS Lett. 580, 6707–6713 (2006).
Dalous, J. et al. Reversal of cell polarity and actin-myosin cytoskeleton reorganization under mechanical and chemical stimulation. Biophys. J. 94, 1063–1074 (2008).
Davis, J. R. et al. Emergence of embryonic pattern through contact inhibition of locomotion. Development 139, 4555–4560 (2012).
Camley, B. A., Zimmermann, J., Levine, H. & Rappell, W.-J. Emergent collective chemotaxis without single-cell gradient sensing. Phys. Rev. Lett. 116, 098101 (2016).
Szabo, A. et al. In vivo confinement promotes collective migration of neural crest cells. J. Cell. Biol. 213, 543–555 (2016).
Berezhkovskii, A. M., Makhnovskii, Y. A. & Suris, R. A. Wiener sausage volume moments. J. Stat. Phys. 57, 333–346 (1989).
Acknowledgements
The authors are grateful to R. Fulcrand for his help in micro-fabrication, to V. Hakim for stimulating discussions and to C. Cottin-Bizonne for her comments on the manuscript. J.d’A. has been partially supported by the Fondation ARC pour la Recherche sur le Cancer and by the Programme d’Avenir Lyon-Saint Étienne. A.S. acknowledges funding through a PLS fellowship of the Gordon and Betty Moor foundation. J.d’A., C.R. and J.-P.R. belong to the CNRS consortium CellTiss and to the LIA ELyTLab.
Author information
Authors and Affiliations
Contributions
J.d’A., J.-P.R. and C.R. designed experiments; J.d’A. performed experiments and analysed experimental data; J.d’A. and A.S. conceived the particle-based models; A.S. performed simulations and analysed simulation data; C.A. contributed to design of experiments in Supplementary Fig. 1 and provided AprA− cells; F.D. computed the analytical results on bimodal trajectories and helped with the fitting procedure; Y.H. assisted in the data analysis and interpretation; J.d’A. and A.S. wrote the manuscript; F.D., J.-P.R. and C.R. made substantial contributions to the manuscript; all authors discussed and interpreted the data, read and commented on the manuscript; J.-P.R. and C.R. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 856 kb)
Supplementary movie
Supplementary movie 1 (MP4 4079 kb)
Supplementary movie
Supplementary movie 2 (MP4 1102 kb)
Supplementary movie
Supplementary movie 3 (MP4 1117 kb)
Supplementary movie
Supplementary movie 4 (MP4 539 kb)
Supplementary movie
Supplementary movie 5 (MP4 3741 kb)
Rights and permissions
About this article
Cite this article
d’Alessandro, J., Solon, A., Hayakawa, Y. et al. Contact enhancement of locomotion in spreading cell colonies. Nature Phys 13, 999–1005 (2017). https://doi.org/10.1038/nphys4180
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys4180
This article is cited by
-
Energetics of mesoscale cell turbulence in two-dimensional monolayers
Communications Physics (2021)
-
Cell migration guided by long-lived spatial memory
Nature Communications (2021)
-
Dynamics of an Expanding Cell Monolayer
Journal of Statistical Physics (2021)
-
Collective migrations in an epithelial–cancerous cell monolayer
Acta Mechanica Sinica (2021)
-
Control of cell colony growth by contact inhibition
Scientific Reports (2020)