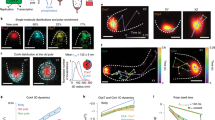
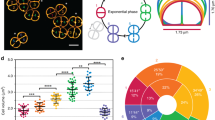
Abstract
Many cellular processes require proteins to be precisely positioned within the cell. In some cases this can be attributed to passive mechanisms such as recruitment by other proteins in the cell or by exploiting the curvature of the membrane. However, in bacteria, active self-positioning is likely to play a role in multiple processes, including the positioning of the future site of cell division and cytoplasmic protein clusters. How can such dynamic clusters be formed and positioned? Here, we present a model for the self-organization and positioning of dynamic protein clusters into regularly repeating patterns based on a phase-locked Turing pattern. A single peak in the concentration is always positioned at the midpoint of the model cell, and two peaks are positioned at the midpoint of each half. Furthermore, domain growth results in peak splitting and pattern doubling. We argue that the model may explain the regular positioning of the highly conserved structural maintenance of chromosomes complexes on the bacterial nucleoid and that it provides an attractive mechanism for the self-positioning of dynamic protein clusters in other systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Murray, J. D. Mathematical Biology II: Spatial Models and Biomedical Applications (Springer, 2003).
Turing, A. M. The chemical basis of morphogenesis. Phil. Trans. R. Soc. Lond. B 237, 37–72 (1952).
Kondo, S. & Miura, T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science 329, 1616–1620 (2010).
Raspopovic, J., Marcon, L., Russo, L. & Sharpe, J. Modeling digits. Digit patterning is controlled by a Bmp-Sox9-Wnt Turing network modulated by morphogen gradients. Science 345, 566–570 (2014).
Levine, H. & Rappel, W.-J. Membrane-bound Turing patterns. Phys. Rev. E 72, 61912 (2005).
Rauch, E. M. & Millonas, M. M. The role of trans-membrane signal transduction in turing-type cellular pattern formation. J. Theor. Biol. 226, 401–407 (2004).
Wingreen, N. S. & Huang, K. C. Physics of intracellular organization in bacteria. Annu. Rev. Microbiol. 69, 361–379 (2015).
Monahan, L. G., Liew, A. T. F., Bottomley, A. L. & Harry, E. J. Division site positioning in bacteria: one size does not fit all. Front. Microbiol. 5, 1–7 (2014).
Rudner, D. Z. & Losick, R. Protein subcellular localization in bacteria. Cold Spring Harb. Perspect. Biol. 2, a000307 (2010).
Thalmeier, D., Halatek, J. & Frey, E. Geometry-induced protein pattern formation. Proc. Natl Acad. Sci. USA 113, 548–553 (2016).
Goryachev, A. B. & Pokhilko, A. V. Dynamics of Cdc42 network embodies a Turing-type mechanism of yeast cell polarity. FEBS Lett. 582, 1437–1443 (2008).
Arcuri, P. & Murray, J. D. Pattern sensitivity to boundary and initial conditions in reaction-diffusion models. J. Math. Biol. 24, 141–165 (1986).
Woolley, T. E., Baker, R. E., Gaffney, E. A. & Maini, P. K. Stochastic reaction and diffusion on growing domains: understanding the breakdown of robust pattern formation. Phys. Rev. E 84, 46216 (2011).
Bard, J. & Lauder, I. How well does Turing’s theory of morphogenesis work? J. Theor. Biol. 45, 501–531 (1974).
Murray, J. D. Parameter space for turing instability in reaction diffusion mechanisms: a comparison of models. J. Theor. Biol. 98, 143–163 (1982).
Maini, P. K., Woolley, T. E., Baker, R. E., Gaffney, E. A. & Seirin Lee, S. Turing’s model for biological pattern formation and the robustness problem. Interface Focus 2, 487–496 (2012).
Loose, M., Kruse, K. & Schwille, P. Protein self-organization: lessons from the min system. Annu. Rev. Biophys. 40, 315–336 (2011).
Nolivos, S. & Sherratt, D. J. The bacterial chromosome: architecture and action of bacterial SMC and SMC-like complexes. FEMS Microbiol. Rev. 38, 380–392 (2014).
Rybenkov, V. V., Herrera, V., Petrushenko, Z. M. & Zhao, H. MukBEF, a chromosomal organizer. J. Mol. Microbiol. Biotechnol. 24, 371–383 (2014).
Cui, Y., Petrushenko, Z. M. & Rybenkov, V. V. MukB acts as a macromolecular clamp in DNA condensation. Nat. Struct. Mol. Biol. 15, 411–418 (2008).
Den Blaauwen, T., Lindqvist, A., Löwe, J. & Nanninga, N. Distribution of the Escherichia coli structural maintenance of chromosomes (SMC)-like protein MukB in the cell. Mol. Microbiol. 42, 1179–1188 (2001).
Ohsumi, K., Yamazoe, M. & Hiraga, S. Different localization of SeqA-bound nascent DNA clusters and MukF-MukE-MukB complex in Escherichia coli cells. Mol. Microbiol. 40, 835–845 (2001).
She, W., Wang, Q., Mordukhova, E. A. & Rybenkov, V. V. MukEF is required for stable association of MukB with the chromosome. J. Bacteriol. 189, 7062–7068 (2007).
Badrinarayanan, A., Lesterlin, C., Reyes-Lamothe, R. & Sherratt, D. J. The Escherichia coli SMC complex, MukBEF, shapes nucleoid organization independently of DNA replication. J. Bacteriol. 194, 4669–4676 (2012).
Badrinarayanan, A., Reyes-Lamothe, R., Uphoff, S., Leake, M. C. & Sherratt, D. J. In vivo architecture and action of bacterial structural maintenance of chromosome proteins. Science 338, 528–531 (2012).
Nolivos, S. et al. MatP regulates the coordinated action of topoisomerase IV and MukBEF in chromosome segregation. Nat. Commun. 7, 10466 (2016).
Ietswaart, R., Szardenings, F., Gerdes, K. & Howard, M. Competing ParA structures space bacterial plasmids equally over the nucleoid. PLoS Comput. Biol. 10, e1004009 (2014).
Wang, H., Wingreen, N. S. & Mukhopadhyay, R. Self-organized periodicity of protein clusters in growing bacteria. Phys. Rev. Lett. 101, 218101 (2008).
Petrushenko, Z. M., Lai, C.-H. & Rybenkov, V. V. Antagonistic interactions of kleisins and DNA with bacterial Condensin MukB. J. Biol. Chem. 281, 34208–34217 (2006).
Crampin, E. J., Gaffney, E. A. & Maini, P. K. Reaction and diffusion on growing domains: scenarios for robust pattern formation. Bull. Math. Biol. 61, 1093–1120 (1999).
Willemse, J., Borst, J. W., de Waal, E., Bisseling, T. & van Wezel, G. P. Positive control of cell division: FtsZ is recruited by SsgB during sporulation of Streptomyces. Genes Dev. 25, 89–99 (2011).
Danilova, O., Reyes-Lamothe, R., Pinskaya, M., Sherratt, D. J. & Possoz, C. MukB colocalizes with the oriC region and is required for organization of the two Escherichia coli chromosome arms into separate cell halves. Mol. Microbiol. 65, 1485–1492 (2007).
Nicolas, E. et al. The SMC complex MukBEF recruits topoisomerase IV to the origin of replication region in live Escherichia coli. MBio 5, e01001-13 (2014).
Wang, X., Possoz, C. & Sherratt, D. J. Dancing around the divisome: asymmetric chromosome segregation in Escherichia coli. Genes Dev. 19, 2367–2377 (2005).
Fisher, J. K. et al. Four-dimensional imaging of E. coli nucleoid organization and dynamics in living cells. Cell 153, 882–895 (2013).
Gruber, S. & Errington, J. Recruitment of condensin to replication origin regions by ParB/SpoOJ promotes chromosome segregation in B. subtilis. Cell 137, 685–696 (2009).
Minnen, A. et al. Control of Smc coiled coil architecture by the ATPase heads facilitates targeting to chromosomal ParB/parS and release onto flanking DNA. Cell Rep. 14, 2003–2016 (2016).
Sullivan, N. L., Marquis, K. A. & Rudner, D. Z. Recruitment of SMC by ParB-parS organizes the origin region and promotes efficient chromosome segregation. Cell 137, 697–707 (2009).
Wang, X., Liu, X., Possoz, C. & Sherratt, D. J. The two Escherichia coli chromosome arms locate to separate cell halves. Genes Dev. 20, 1727–1731 (2006).
Bates, D. & Kleckner, N. Chromosome and replisome dynamics in E. coli: loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell 121, 899–911 (2005).
Nielsen, H. J., Li, Y., Youngren, B., Hansen, F. G. & Austin, S. Progressive segregation of the Escherichia coli chromosome. Mol. Microbiol. 61, 383–393 (2006).
Lau, I. F. et al. Spatial and temporal organization of replicating Escherichia coli chromosomes. Mol. Microbiol. 49, 731–743 (2004).
Lim, H. C. et al. Evidence for a DNA-relay mechanism in ParABS-mediated chromosome segregation. eLife 2014, e02758 (2014).
Hu, L., Vecchiarelli, A. G., Mizuuchi, K., Neuman, K. C. & Liu, J. Directed and persistent movement arises from mechanochemistry of the ParA/ParB system. Proc. Natl Acad. Sci. USA 112, E7055–E7064 (2015).
Halley, J. D. & Winkler, D. A. Consistent concepts of self-organization and self-assembly. Complexity 14, 10–17 (2008).
Roberts, M. A. J., Wadhams, G. H., Hadfield, K. A., Tickner, S. & Armitage, J. P. ParA-like protein uses nonspecific chromosomal DNA binding to partition protein complexes. Proc. Natl Acad. Sci. USA 109, 6698–6703 (2012).
Treuner-Lange, A. et al. PomZ, a ParA-like protein, regulates Z-ring formation and cell division in Myxococcus xanthus. Mol. Microbiol. 87, 235–253 (2013).
Dillon, R., Maini, P. K. & Othmer, H. G. Pattern formation in generalized Turing systems. J. Math. Biol. 32, 345–393 (1994).
Matoba, K., Yamazoe, M., Mayanagi, K., Morikawa, K. & Hiraga, S. Comparison of MukB homodimer versus MukBEF complex molecular architectures by electron microscopy reveals a higher-order multimerization. Biochem. Biophys. Res. Commun. 333, 694–702 (2005).
Gloyd, M., Ghirlando, R. & Guarné, A. The role of MukE in assembling a functional MukBEF complex. J. Mol. Biol. 412, 578–590 (2011).
Crampin, E. J. & Maini, P. K. Reaction-diffusion models for biological pattern formation. Methods Appl. Anal. 8, 415–428 (2001).
Gillespie, D. T. Stochastic simulation of chemical kinetics. Annu. Rev. Phys. Chem. 58, 35–55 (2007).
Gibson, M. & Bruck, J. Efficient exact stochastic simulation of chemical systems with many species and many channels. J. Phys. Chem. A 104, 1876–1889 (2000).
Cao, Y., Li, H. & Petzold, L. R. Efficient formulation of the stochastic simulation algorithm for chemically reacting systems. J. Chem. Phys. 121, 4059–4067 (2004).
Riaz, S. S., Sharma, R., Bhattacharyya, S. P. & Ray, D. S. Instability and pattern formation in reaction-diffusion systems: a higher order analysis. J. Chem. Phys. 127, 64503 (2007).
Wei, J. & Winter, M. Mathematical Aspects of Pattern Formation in Biological Systems. Applied Mathematical Sciences Vol. 189 (Springer, 2014).
Bakshi, S. et al. Nonperturbative imaging of nucleoid morphology in live bacterial cells during an antimicrobial peptide attack. Appl. Environ. Microbiol. 80, 4977–4986 (2014).
Sliusarenko, O., Heinritz, J., Emonet, T. & Jacobs-Wagner, C. High-throughput, subpixel precision analysis of bacterial morphogenesis and intracellular spatio-temporal dynamics. Mol. Microbiol. 80, 612–627 (2011).
Wallden, M., Fange, D., Lundius, E. G., Baltekin, Ö & Elf, J. The synchronization of replication and division cycles in individual E. coli cells. Cell 166, 729–739 (2016).
Acknowledgements
We thank D. Sherratt for providing strain AB45 and R. Colin, U. Endesfelder, P. Graumann, M. Howard, S. Jun and A. Paulick for discussions and/or comments on the manuscript. This work was supported by the German Federal Ministry of Education and Research and the Max Planck Society in the framework of the research network MaxSynBio.
Author information
Authors and Affiliations
Contributions
S.M.M. initiated the work, conceived the model and designed and performed simulations and experiments. V.S. contributed to experiment design. Both authors discussed the results and implications. S.M.M. wrote the initial draft of the manuscript. Both authors edited subsequent and final versions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1973 kb)
Rights and permissions
About this article
Cite this article
Murray, S., Sourjik, V. Self-organization and positioning of bacterial protein clusters. Nature Phys 13, 1006–1013 (2017). https://doi.org/10.1038/nphys4155
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys4155
This article is cited by
-
Isolating Patterns in Open Reaction–Diffusion Systems
Bulletin of Mathematical Biology (2021)
-
Nucleoid-mediated positioning and transport in bacteria
Current Genetics (2020)